Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Fuel-Cell Membrane Degradation
Theoretical study points to destructive role of radical species
by Jyllian Kemsley
October 31, 2011
| A version of this story appeared in
Volume 89, Issue 44
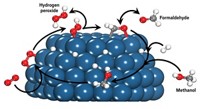
Degradation of Nafion, a common fluoropolymer membrane used to separate electrodes and reactants in fuel cells, involves the reaction of H2, O2, and platinum to produce radical species that then attack the intact polymer rather than defect sites, reports a group led by Caltech’s William A. Goddard III (J. Am. Chem. Soc., DOI: 10.1021/ja2074642). Understanding how Nafion degrades is key to finding ways to make fuel cells work longer. Researchers have long believed that radical species play a role in Nafion damage, but how the radicals arise and react with the polymer has been uncertain. Using a theoretical analysis, Goddard and colleagues found that HO∂ forms from H2O2 produced at the surface of Pt, which is used as a catalyst in fuel cells. The researchers then observed that HO∂ can degrade the polymer in two ways: either by attacking C–S bonds or by reacting with H2 to form H∂, which attacks C–F bonds. The results point to ways to prevent membrane damage, such as by modifying catalysts to prevent H2O2 formation or creating polymers that are more resistant to radical attack, the authors say.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter