Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Cage-Shaped Borate Catalyst Recognizes Aromatic Aldehydes
Lewis acid catalyst with an enzymelike binding pocket selects aromatic aldehydes over aliphatic aldehydes in Diels-Alder reactions
by Stephen K. Ritter
March 19, 2012
| A version of this story appeared in
Volume 90, Issue 12
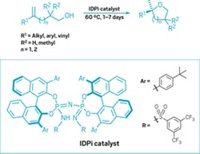
A boron-based organocatalyst with an enzymelike π-binding pocket has been designed to catch and hold aromatic aldehydes to facilitate Diels-Alder cycloaddition reactions. The research team led by Makoto Yasuda and Akio Baba of Japan’s Osaka University believes this is the first reported example of the molecular recognition of an aromatic aldehyde over an aliphatic aldehyde in a catalytic manner. Yasuda, Baba, and coworkers previously designed cage-shaped triphenylborate Lewis acids, such as B(OC6H4)3CH, that allow them to increase Lewis acidity over planar, open-shaped borate catalysts. The triphenylborate has a shallow binding pocket and reacts nearly equally with aromatic and aliphatic aldehydes, which the team showed in competitive Diels-Alder reactions pitting benzaldehyde against butanal. The researchers have now taken the borate catalysts to a different level by adding aryl groups to the phenyl rings to form B(OC6H3aryl)3CH analogs, such as the naphthyl example shown (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201200346). The additional aryl rings create a deeper pocket and a larger π-aromatic framework for the catalyst to selectively bind aromatic aldehydes. The naphthyl catalyst favors p-cyanobenzaldehyde over butanal at a ratio of up to 27.5:1.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter