Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Surface Do-Overs
Photochemistry: Rapid reaction attaches protein or dyes
by Carmen Drahl
May 28, 2012
| A version of this story appeared in
Volume 90, Issue 22

Using ultraviolet light, chemists have reversibly immobilized molecules on surfaces (J. Am. Chem. Soc., DOI: 10.1021/ja302970x). The technique could be used to develop repairable microarrays or to study how biochemical events on surfaces progress with time.
Light-mediated reactions are nothing new when it comes to decorating surfaces for bioanalytical chemistry. But most techniques are irreversible or make it difficult to reuse a surface.
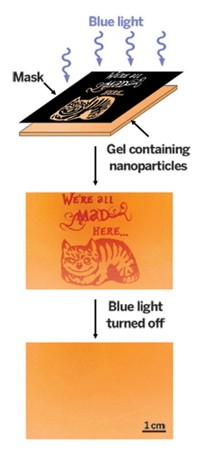
Now, postdoctoral researcher Selvanathan Arumugam and associate professor Vladimir V. Popik of the University of Georgia have reversibly modified thiol-coated glass slides with 2-naphthoquinone-3-methides, which they generate from a naphthol precursor by illumination with 350-nm-wavelength light from a conventional thin-layer chromatography lamp. By selectively blocking light exposure with a transmission electron microscopy grid, they patterned slides with a naphthol connected to a fluorescent dye. Exposing patterned slides to the same wavelength of light releases the naphthol, regenerating surface thiols.
The reaction is compatible with azide-alkyne click chemistry, which the team used in two-step protocols to pattern slides with another dye or the protein avidin. The team was also able to replace one immobilized molecule with another, which could make it possible to repurpose microarray technologies in addition to repairing them.
“We use glass slides to demonstrate the procedure—we could use any surface with thiols,” Popik says. Plans to functionalize metal nanoparticles and proteins are in the works, he adds. The University of Georgia has filed a provisional patent on the technology.
The team’s big insight was developing a surface modification method that generates the reactive intermediate in solution instead of on the surface itself, says Craig J. Hawker, who designs structures for surface science at the University of California, Santa Barbara. That allows by-products to form in solution instead of squandering precious real estate on the surface, he explains. If follow-up work leads to even better spatial control and the ability to perform the chemistry on varied surfaces, he thinks the technology could be widely adopted.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter