Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Small Molecule Enables Blind Mice To Respond To Light
Drug Discovery: Research paves way for drug treatment of degenerative blindness
by Deirdre Lockwood
July 30, 2012
| A version of this story appeared in
Volume 90, Issue 31
\
\
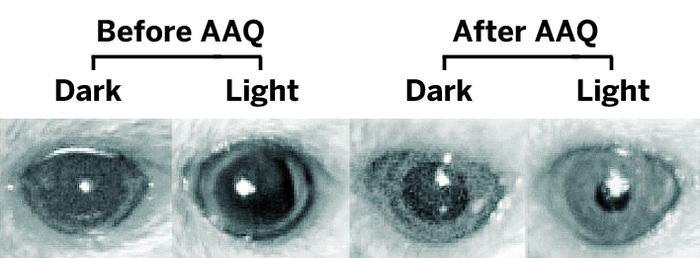
Light reflex in the eyes of blind mice can be restored when a potassium channel is unblocked by the light-sensitive small molecule AAQ.
A small-molecule “photoswitch” has enabled blind mice to respond to light (Neuron, DOI: 10.1016/j.neuron.2012.05.022). The work could pave the way for drugs to treat degenerative blindness, which affects 1 in 3,000 people worldwide.
Drugs would be a promising treatment for degenerative blinding diseases such as age-related macular degeneration and retinitis pigmentosa, which ensue when the eye’s light-sensing machinery dies. In contrast, alternative therapies in development, such as prosthetic retinal chips and gene therapy, involve invasive or irreversible intervention.
When researchers injected the chemical into the eyes of genetically modified blind mice and shined light on them, the mice ran away, in a classic avoidance response. Their pupils also contracted in the presence of light, in contrast to remaining dilated without the compound. The effect wore off after 24 hours.
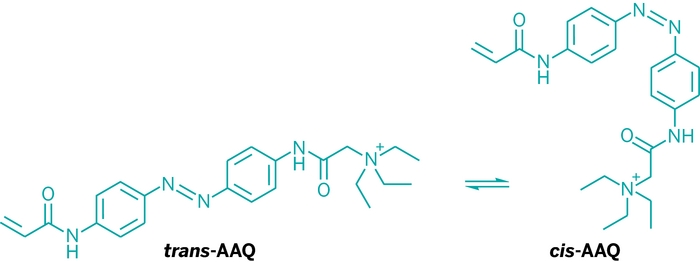
Researchers, including Richard H. Kramer, a neurobiologist at the University of California, Berkeley, and Dirk Trauner, a chemical biologist at the University of Munich, developed the photoswitch molecule, acrylamide-azobenzene-quaternary ammonium, or AAQ. The compound makes an ion channel sensitive to light through a mechanism similar to that of the painkiller lidocaine. In the dark, AAQ is in its trans form, which blocks a potassium channel, but in white light it isomerizes to its cis form, unblocking the channel and allowing specific retinal neurons to fire, sending visual input to the brain.
“It is a very important and cool step forward that may bypass the need for complex viral therapies to deliver genes of frankly unknown efficacies to human eyes,” says Robert E. Marc, director of research at the University of Utah’s department of ophthalmology and visual sciences. “We haven’t had anything like it.”
Trauner says the study provides an exciting first proof of principle, but “we’re still far from knowing whether this could restore vision.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter