Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Borenium Salt Catalysts Readily Reduce Imines
Lewis acid-base pair complexes couple with a neutral borane to create highly reactive organocatalysts
by Stephen K. Ritter
October 15, 2012
| A version of this story appeared in
Volume 90, Issue 42
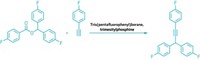
Canadian chemists have learned how to generate powerful metal-free catalysts for important imine reduction reactions by starting from boron-based Lewis acid-base complexes (J. Am. Chem. Soc., DOI: 10.1021/ja307374j). Patrick Eisenberger, Adrian M. Bailey, and Cathleen M. Crudden of Queen’s University, in Kingston, Ontario, coupled B(C6F5)3 or [(C6H5)3C][B(C6F5)4] with 1,4-diazabicyclo[2.2.2]octane and pinacolborane. The resulting catalytic salts consist of a highly electrophilic borenium cation paired with a fluorophenylborate anion (one shown). Crudden’s team found that the catalytic borenium complexes activate the C=N bond of a broad range of imines and use the hydrogen from pinacolborane to reduce the C=N bond to form secondary amines under mild reaction conditions. In another paper just published, Jeffrey M. Farrell, Jillian A. Hatnean, and Douglas W. Stephan of the University of Toronto report a related borenium catalyst that activates and uses H2 for imine reductions (J. Am. Chem. Soc., DOI: 10.1021/ja307995f). These two reports are the first examples of borenium catalysts being used for metal-free reductions, Crudden says. Both groups provide new strategies for avoiding the use of stoichiometric borohydride salts, but Crudden’s version offers a unique mechanism that also avoids the need to use H2 gas.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter