Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Shifting Market For Repellency Chemicals
Regulators and environmentalists force changes in the water repellency business
by Michael McCoy
December 3, 2012
| A version of this story appeared in
Volume 90, Issue 49

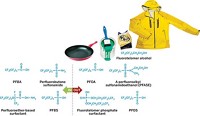
Fluorine chemical companies have been selling short. To please the Environmental Protection Agency, the firms are pushing textile industry customers to change a key ingredient in water- and oil-repellent treatments from a toxic long-chain fluorinated compound to a shorter one.


But the environmental group Greenpeace is shaking up this happy cooperation by questioning the use of all fluorinated chemicals, long and short, in the textile industry. In a new report, the group argues that all such compounds are problematic and demands that regulators scrutinize them for a possible ban. Greenpeace wants the textile industry to switch to completely nonfluorinated alternatives.
The campaign is having an impact. In recent weeks, two major clothing retailers announced that they plan to remove all fluorinated treatment chemicals from their product lines. Meanwhile, firms in the outdoor-clothing sector are redoubling their efforts to switch to short-chain compounds. The textile industry, once a stable market for repellency chemicals, is splintering.
EPA sought a phaseout of the long-chain compounds—mainly eight-carbon perfluorinated chemicals (PFCs)—not only because laboratory tests showed they are toxic to animals but also because they persist in the environment. The chemical companies are obliging because they say the shorter-chain compounds, mostly six carbons long, are better for the environment and do just as good a job.
The agency began its efforts in 2000 after it became concerned about the presence of one particular eight-carbon compound, perfluorooctanoic acid (PFOA), in the blood of most Americans.
In 2006, EPA got eight major fluorochemical producers to agree to a 95% reduction, by 2010, in factory emissions and end-product content of both PFOA and chemicals that can break down into PFOA. The firms also pledged to work toward the complete elimination of these chemicals from emissions and end products by 2015.
The companies were confident in their ability to fulfill the pledge because they knew that products based on shorter six-carbon PFCs didn’t involve PFOA and could be designed to repel oil and water just as well.
For example, DuPont, a fluorochemistry pioneer, has long manufactured both C6 and C8 PFCs as ingredients in its Teflon-brand water, oil, and stain repellents, according to Thomas H. Samples, global business manager for the firm’s surface protection unit.
For certain applications, repellency products with a six-carbon fluorinated chain were always more effective than their eight-carbon counterparts, according to Samples. DuPont began to explore replacing its C8 products around 2004, he says, and by 2006 it had determined that customers could be transitioned to C6 chemistry. DuPont started the process with stain and soil repellency products for the carpet industry and moved on through textiles and other markets.
Today, DuPont’s key repellency building block is a fully fluorinated six-carbon chain connected to a two-carbon alcohol. This fluorinated alcohol can be functionalized to allow grafting to a polymer that is compatible with the fiber or textile to be treated. The vast majority of DuPont’s customers are already using C6 chemistry, Samples says, and the company expects to complete the switch by EPA’s 2015 deadline.
Also making the transition is the Swiss specialty chemical maker Clariant, which for years has manufactured long-chain PFCs in Gendorf, Germany.
Georg Lang, head of the finishing products group in Clariant’s textile chemicals unit, points out that his company neither makes nor uses PFOA. However, the chemical can be created as a less-than-1-ppm by-product during long-chain PFC manufacturing, he says, and it might be created as a breakdown product of C8 PFCs in the environment.
Soon after EPA voiced its initial concerns about long-chain PFCs, Clariant began researching shorter-chain products based on a six-carbon backbone, Lang says. The effort paid off. “We were able to bring our first really well performing C6 water- and oil-repellent product to market in 2007,” he says.
Clariant’s initial C6 production was in existing reactors at the Gendorf plant, but output was limited. Two months ago, the company announced the completion of a $10 million project to increase C6 capacity at the site to support customers that are switching from C8 chemistry. The majority of Clariant’s customers are still using C8 products, Lang says, but the balance has been moving steadily, and he expects the shift to accelerate.
As it increased capacity, Lang says, Clariant was also working to improve the performance of short-chain water repellency products. Today, in markets such as apparel, he says, the C6 chemicals perform as well as the C8 ones. In specialized applications such as outdoor awnings, he acknowledges, “they aren’t there quite yet.”
Some clothiers, however, are seeking to avoid PFCs altogether. In September the Swedish clothing chain H&M said it is putting a global ban on PFCs in all of its products. All apparel that it orders after Jan. 1, 2013, the company declared, must be produced without PFCs.
In October, the British retailer Marks & Spencer published a set of chemical commitments drawn up in consultation with Greenpeace. The commitments reinforced M&S’s ban on alkylphenol ethoxylate surfactants in its products and set up a phased elimination of PFCs from its goods as well.
As of Jan. 1, 2013, M&S is banning C8 PFCs. By the end of 2013, it plans to have achieved a 50% reduction in the number of products it sells with PFCs of any kind, and by July 1, 2015, it aims to have implemented a 90% reduction in products with PFCs.
A week after M&S stated its intentions, Greenpeace came out with its report on the use of fluorinated chemicals by outdoor clothing manufacturers. Called “Chemistry For Any Weather,” the report is part of Greenpeace’s Detox campaign to improve the environmental impact of textile production. It argues that short-chain PFCs are as persistent as PFOA in the environment and can reach groundwater more easily because they don’t bond as well to their substrates.
For the report, Greenpeace commissioned two independent labs to test weatherproof jackets and pants. PFCs were found in all 14 products tested; in five of them, the group concluded, PFOA was present in significant concentrations. They were jackets made by Jack Wolfskin, Patagonia, The North Face, and Kaikkialla and children’s pants from Marmot.
Given their connection to people’s outdoor recreation, companies in the outdoor-clothing business are sensitive to accusations of environmental damage. In September, Jack Wolfskin, a German clothing maker, vowed that all of its weather protection products would be made without PFOA by the end of 2014. The firm said it has been moving in that direction since 2009.
Patagonia acknowledged last month that it does use C8 PFCs in its waterproof garments. The company says it will have converted about 40% of its products to C6 technology by the coming spring and its entire line by the fall of 2015. “We’ve worked with chemical companies to suggest and test improvements in the repellency and durability of both C6 and fluorocarbon-free finishes,” the firm says.
In a position paper on the topic, Patagonia argues that a fluorocarbon-free water repellent would be preferable to removing individual fluorochemicals as potential health and safety concerns are identified. But the company points to shortcomings in fluorine-free products that have kept it from adopting them to date.
Indeed, pretty much everyone in the business of repellents for textiles agrees that fluorine-free chemistry is inferior to fluorine-based chemistry because it is ineffective at repelling alcohols and oils. Samples notes that fluorine-based products like DuPont’s sell well even though they are typically more expensive than alternatives based on silicones or hydrocarbons.
“Many applications where water repellency can be achieved by nonfluorine means were done years ago because of cost,” he says. Companies are now revisiting how much performance they require, he adds. “Where they need the performance, they are going to use short-chain fluorinated compounds.”
Patagonia points out that an effective and durable product, even if made with fluorinated chemicals, is an environmentally sound product. “The longer a waterproof jacket remains waterproof, the longer it stays out of a landfill,” the firm says. “This is very important to us.”
Still, chemical companies continue to pursue fluorine-free waterproofing techniques. Early this year the Swiss textile treatment firm Schoeller Technologies launched Ecorepel, a repellent based on paraffin chains that, according to the company, “wrap themselves as a spiral around the individual fibers, filaments, or yarns in a very fine film” so that water and even watery mud run off.
In March, Dow Corning came out with DWR-7000 Soft Hydro Guard, a silicone-based emulsion that it claims offers water repellency on a par with fluorocarbon technologies. Its silicones competitor Momentive Performance Materials offers a two-component repellency system containing a silicone emulsion and a curing catalyst.
Clariant’s fluorine-free entry, launched in June, is called Arkophob FFR. A chemically modified paraffin, the new product is superior to existing fluorine-free alternatives, Clariant claims, and performs almost as well as C6 PFCs as a water repellent. It holds up after more than 20 washes, also close to the performance of C6 products, the firm says.
Yet Lang freely admits that the Arkophob doesn’t offer the oil and stain repellency of fluorine-based products. “If you want to have repellency not only against water but also against liquid soils and oils, you have to stick with fluorocarbon chemistry,” he says.
Mark Brutten tells a similar story. Brutten is senior vice president for sales and marketing at Nano-Tex, a California-based textile finishing specialist that last year launched Aquapel, a hydrocarbon polymer with chains consisting of “hooks” that attach to fabric and “whiskers” that repel water.
“We promote it as being ideal for protection against the elements,” Brutten says. “It’s specifically targeted at the outdoor market.” One customer is the Dick’s Sporting Goods chain, which adopted Aquapel earlier this year for its Köppen line of clothing for paddle sports. Still, Nano-Tex does not promote Aquapel for stain or oil repellency.
Nano-Tex was also an early adopter of C6 PFCs, Brutten says, launching its first products five years ago. Today, Brutten estimates, 75% of Nano-Tex’s repellency customers use C6 products and 15% use C8 products. Only about 10% use Aquapel, he says, even though it is as effective as fluorinated products in water repellency and costs 30–40% less.
In Brutten’s view, H&M and Marks & Spencer are anomalies in the retail world. Greenpeace aside, he says textile sustainability organizations such as Bluesign Technologies approve of the switch to C6 PFCs, and he expects PFCs to be the dominant repellency approach for the foreseeable future.
Not surprisingly, DuPont’s Samples feels the same way. Yet he also doesn’t discount the possibility of breakthroughs in nonfluorine chemistry that may someday alter the repellency landscape.
“The industry has to continue to move,” Samples says. “It’s a good thing for us to try to provide better chemistry that meets the performance requirements of our customers and improves the profile for the consumer and the world. We’re on board with that.” ◾



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter