Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Peroxide Dianion Finds A Stable Home
Using hydrogen bonds to stabilize peroxide dianion in a macrocycle provides a metal-free source of a useful oxidant
by Stephen K. Ritter
January 30, 2012
| A version of this story appeared in
Volume 90, Issue 5
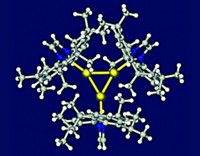
Like a hermit crab occupying an abandoned seashell, the transient peroxide dianion (O22–) has been enticed by researchers at Massachusetts Institute of Technology to take up residence in a hydrogen-bonding macromolecule (Science, DOI: 10.1126/science.1212678). Generating stable, soluble sources of O22– has historically been a challenge in dioxygen chemistry. Reduction of O2 to O22– is typically carried out in chemical and biochemical oxidation processes by transition-metal complexes. Nazario Lopez, Christopher C. Cummins, Daniel G. Nocera, and coworkers hypothesized that O22– could be stabilized without a metal if it were surrounded by a hydrogen-bonding environment. The team found that the hexacarboxamide cryptand fit the bill, serving as an anion receptor for O22–. The researchers prepared gram quantities of cryptand-encapsulated O22– either by the disproportionation of KO2 or by the reduction of O2 by cobaltocene. The dianion is stabilized by a combination of six strong hydrogen bonds to the cryptand’s six amide hydrogen atoms (shown) and by six weak hydrogen bonds to the cryptand’s three aryl hydrogen atoms (not shown). The researchers used electrochemical experiments and simulations to understand how the mechanism of O2 reduction at an electrode surface is profoundly altered in the presence of the cryptand.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter