Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Zeolite Mixes Up Pore Sizes To Good Effect
Material with 10- and 12-sided channels converts naptha to diesel fuel with minimal carbon fouling
by Jyllian Kemsley
January 30, 2012
| A version of this story appeared in
Volume 90, Issue 5
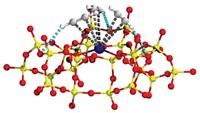
A complex zeolite with differently sized, interlocking pores shows promise for converting naphtha to diesel fuel while avoiding fouling by residual carbon that can sideline some catalysts (Nat. Chem., DOI: 10.1038/nchem.1253). Aluminosilicate zeolites with pores bounded by either 10 or 12 silicon or aluminum atoms are important petrochemical catalysts, raising interest in a material that combines the two pore sizes. A group led by Xiaodong Zou of Stockholm University and Avelino Corma of Polytechnic University of Valencia, in Spain, has synthesized just such a zeolite and solved its structure using electron crystallography of nanometer-sized crystals. Called ITQ-39, the material contains intersecting 10- and 12-ring channels that run perpendicular to each other. The diameters of the channels are about 6 Å and 7 Å, respectively. In tests of naphtha alkylation, ITQ-39 performed better than commercial zeolite catalysts.The researchers believe the 12-ring channels promote the formation and diffusion of alkylated products, whereas the 10-ring channels prevent coke deposits by precluding the formation of polyaromatic precursor molecules.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter