Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Organic Synthesis: One-Step Arylboronates
Two research teams independently reported iridium catalysts, leading to an abbreviated version of the Suzuki coupling reaction
by Bethany Halford
December 24, 2012
| A version of this story appeared in
Volume 90, Issue 52
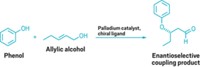
When it comes to assembling molecules, synthetic chemists find that the Suzuki coupling reaction is as indispensable to them as a screwdriver is to a carpenter. A 2011 review article estimates that the method, which traditionally cross-couples an organoboron compound with an organohalide to form a new carbon-carbon bond, accounts for 40% of all C–C bond-forming reactions used in the pursuit of drug candidates (J. Med. Chem., DOI: 10.1021/jm200187y). Its utilitarian nature earned its discoverer, Akira Suzuki of Hokkaido University in Japan, a share of the 2010 Nobel Prize in Chemistry.
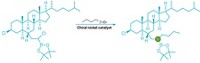
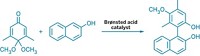
But even a great reaction can suffer from tough-to-obtain reagents. So in 2002 when two groups independently announced they had discovered iridium catalysts that turn arenes into arylboronic esters in one step, as opposed to traditional multistep approaches, it was as if chemists had been handed a cordless electric screwdriver.
The reaction gives chemists a tool for taking a C–H bond and turning it into something that can readily undergo a subsequent chemical transformation, says Milton R. Smith III of Michigan State University, who led one of the research efforts (Science, DOI: 10.1126/science.1067074).
“The key to this chemistry is that it allows us to get to these compounds much more efficiently,” adds Robert E. Maleczka Jr., Smith’s Michigan State collaborator. “It allows access to many sorts of substitution patterns that were traditionally unavailable. It really opens up the opportunity to make new building blocks.”
The Michigan State chemists took advantage of the chemistry to start BoroPharm, a company that sells boronic acids and related chemicals. Smith and Maleczka also received a 2008 Presidential Green Chemistry Challenge Award for their work.
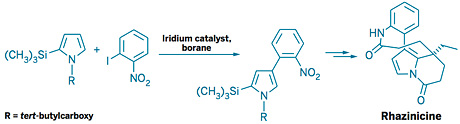
John F. Hartwig, then of Yale University, whose team independently developed an iridium catalyst for the transformation, notes that the reaction has been widely used in academic and industrial laboratories. “One medicinal chemist stated to me that this reaction has become part of their regular repertoire,” says Hartwig, now at the University of California, Berkeley. He carried out the original work with Hokkaido chemists Tatsuo Ishiyama and Norio Miyaura (J. Am. Chem. Soc., DOI: 10.1021/ja0173019).
“It is remarkable that only about a decade separates the initial observation of stoichiometric functionalization of arenes and alkanes with metal-boryl complexes in high yield and the development of catalysts for the borylation of arenes that are now widely used,” Hartwig and colleagues noted in a 2010 review (Chem. Rev., DOI: 10.1021/cr900206p).
Tweaking the catalyst ligands over time has transformed the chemistry to make it even more powerful, Hartwig adds. “If the two catalysts the two groups published in 2002 were all that were available, the synthetic community would never be using this chemistry as commonly as they use it today,” he says.
The iridium-catalyzed chemistry is now being used to synthesize natural products and fine chemicals, as well as in materials science. The reaction takes place under mild conditions and tolerates a broad range of functional groups. “These reactions are inherently clean, with hydrogen gas being the only stoichiometric by-product,” Maleczka points out. “The functional group tolerance really set the stage for us to be able to combine these reactions with subsequent chemical events, so you can do multiple steps in one pot.”
“I think there’s still a lot that’s going to be developed in the future on this,” Smith adds. “In 10 years there are likely to be more advances that will put this into even more people’s hands.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter