Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Periodic Table
The Human Element
Discovery of the final seven naturally occurring elements reveals the complex human interactions that drive science
by Sam Lemonick
June 24, 2013
| A version of this story appeared in
Volume 91, Issue 25
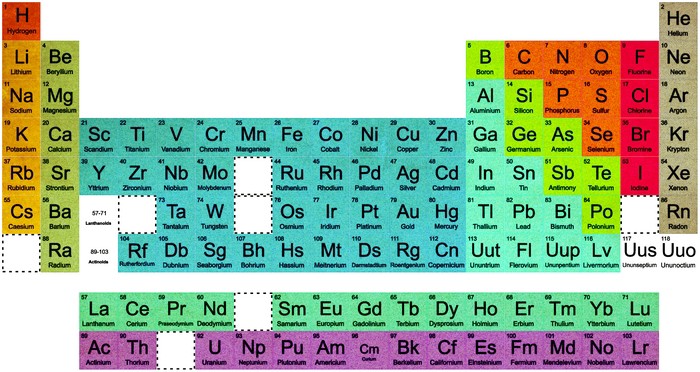
“In 1492, Columbus sailed the ocean blue,” and discovered America. Or rather, he was the first European to reach America, where millions of people already lived. And actually some Norsemen had been to the continent before him anyway. That’s how tidy stories in history tend to fall apart, and a new book by Eric Scerri shows that the history of chemistry is no different.
Scerri’s “A Tale of Seven Elements” tells the story of how the last seven elements between hydrogen and uranium—technetium, promethium, hafnium, rhenium, astatine, francium, and protactinium—were discovered. By virtue of their rarity, their short life spans, or their affinity for similar elements, chemists struggled for decades to isolate each of them. Scerri ably describes these laboratory trials and tribulations, but the heart of his story is the messiness of the human side of science and how that has shaped the periodic table that we all recognize.
Egos, nationalism, and some plain bad luck have a lot to do with whom we credit for discovering an element. In some cases, the first claimant turns out to have been mistaken. In others, the perspective of a half-century has restored to a degree the primacy of one claim or another. While the periodic table may look irrefutable hanging in a lecture hall, the stories of these seven elements show how fluid it can sometimes be.

Scerri devotes the first quarter of the book to the history of the periodic table from its invention, through the evolutions in its organization, to what he calls the invasion of physics. The discovery of electrons, isotopes, and quantum theory by physicists clarified and affirmed the way chemists had arranged the elements. Much of this section seems distilled from Scerri’s earlier book “The Periodic Table: A Very Short Introduction,” which would be an excellent place to go next if you’re intrigued by the ideas here.
Beginning with Mendeleev’s periodic table, usually thought of as the first, Scerri is quick to show how history can either sanitize or clarify what really happened. In fact, scientists like Emile de Chancourtois, William Odling, and Julius Lothar Meyer can all claim to have invented a periodic system during the mid-1800s, although Scerri makes clear that some were better than others. Nonetheless, he explains that Mendeleev’s fame is based as much on circumstances as on the validity of his table and his predictions for undiscovered elements.
Chemists of the time looked down on de Chancourtois, for instance, because he was trained as a geologist. Even still, his ideas might have caught on if the journal he published his seminal paper in had included the diagram of the cylindrical periodic system he had devised. Scerri unpacks these dramatic periods for his reader to show both how it happened and how they came to be remembered as they are.
After this condensed lesson on the periodic table, Scerri spends the rest of the book on the titular seven elements, each with its own chapter in the order of their discoveries. Like the book’s first quarter, these chapters are broken up into shorter sections that deal with a particular individual or event. Scerri works hard to expose all the complexity of discovering a single element, and unfortunately there are places where that muddle pollutes the text, making the narrative disjointed and a little confusing. I wish Scerri had had twice as many pages to tell this tale.
Despite that, these are fascinating stories. The common response to the complaint “chemistry is boring” is an exploding balloon of hydrogen or something similar, but the human element that Scerri describes is much more interesting. Take the controversy over element 72, now called hafnium, which Scerri calls “one of the most bitter and acrimonious priority disputes in the twentieth century.”
Element 72 was among those predicted by Mendeleev, in the form of a blank space in his original 1869 table. In 1911, a French chemist and expert on rare-earth metals named Georges Urbain announced he had isolated the element, which he called celtium. A newly developed X-ray method gave conflicting results about the veracity of his claim.
Meanwhile, the physicist Niels Bohr declared on the basis of atomic theory that 72 should not be a rare earth at all. In 1922, two researchers at his institute in Copenhagen, Dirk Coster and Georg Hevesy, used the same X-ray method to declare they had found the element and to discredit Urbain on the basis of Bohr’s reasoning. With Europe just emerging from the shadow of World War I, their paper set off a nasty battle of words. French and English scientists sided with Urbain out of nationalistic loyalty, and each side referred to the element by their own name. After accusations of plagiarism and some maneuvering by Urbain and company, Coster and Hevesy prevailed and element 72 is called hafnium after the Latin name for Denmark.
The flip side of that example is something like mendelevium, which is not one of the seven elements but shows up in the last chapter on the synthetic transuranium elements. Produced at the University of California, Berkeley, during the Cold War, its creators named it after Mendeleev, a Russian, in a rare moment of friendship between the hostile nations.
One nice touch is an explanation of the modern uses for each of the seven elements. The compound rhenium boride is hard enough to cut diamond, for instance, whereas technetium is used for medical imaging. The sections provide useful reference points for readers who feel like the subject matter is a little too abstract.
Priority disputes like the one over hafnium were the rule, not the exception, for Scerri’s seven elements, and some were even lengthier and more complex. And at their root are men and women—many women, in fact, whom Scerri very admirably champions—whose virtues and failings have as much to do with the periodic table we know as they do with X-ray spectrometers and cyclotrons.
In his introduction, Scerri writes that the conflicts over priority “are revealing of the often frail humanity of the scientists involved or perhaps of the scientific method as a whole and the pressures which it often places on scientists.” “A Tale of Seven Elements” reveals that conscientiously and fully.
Sam Lemonick is a freelance science writer in Washington, D.C.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter