Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Adding Unnatural Covalent Bond To Proteins
Unnatural amino acid reacts with cysteine side chain
by Celia Henry Arnaud
August 12, 2013
| A version of this story appeared in
Volume 91, Issue 32
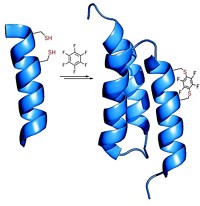
Covalent bonds between amino acid side chains help stabilize protein structures and interactions. The ability to form types of covalent bonds other than disulfide bonds between cysteines could make it possible to design proteins with a wider variety of properties and functions. Lei Wang and coworkers at the Salk Institute for Biological Studies, in La Jolla, Calif., use well-established methods to incorporate into proteins unnatural amino acids that form covalent bonds not normally seen in proteins (Nat. Methods 2013, DOI: 10.1038/nmeth.2595). Researchers have previously avoided unnatural amino acids that react with other side chains, opting instead for so-called bioorthogonal side chains. Wang and coworkers designed p-2´-fluoroacetylphenylalanine to react with cysteine (reaction shown). The reaction occurs only when the two amino acids are close to one another in the same protein or interacting proteins. The researchers show that when the unnatural amino acid is incorporated in an antibody mimetic, it enables irreversible binding between the mimetic and its protein substrate. They suspect the approach will also work with unnatural amino acids designed to react with amino acids other than cysteine.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter