Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Enantioselective Photocycloadditions
Chiral Lewis acid guides enone in intramolecular reaction
by Bethany Halford
November 18, 2013
| A version of this story appeared in
Volume 91, Issue 46
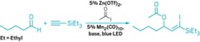
The light-activated cycloaddition reaction that weds the C=C bond of an enone with another alkene group to create a four-membered ring has been around for more than 100 years. Despite its age, chemists have found it challenging to create an enantioselective version of this transformation. In the past, chiral auxiliaries—chemical compounds that are temporarily added to the reactants—have been used, but attaching and removing these units require extra steps. Now, Thorsten Bach and Richard Brimioulle of Technical University of Munich have found a simpler way to guide the stereochemical outcome of this reaction (Science 2013, 10.1126/science.1244809). In the presence of a chiral Lewis acid and UV light, 5,6-dihydro-4-pyridones cleanly undergo enantioselective intramolecular cycloadditions (shown). Reported yields are fair to good, with enantiomeric excesses of 82–90%. The researchers used the reaction to synthesize two natural products, (+)-lupinine and (+)-thermopsine. This strategy may provide a general approach to similar enantioselective cycloadditions of enones, the researchers say.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter