Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Art In Science: Surface Features Make Captivating Images
Switchable alkanethiol molecules and chiral metal oxide films added aesthetic beauty to chemical science
by Stephen K. Ritter
December 23, 2013
| A version of this story appeared in
Volume 91, Issue 51

COVER STORY
Art In Science: Surface Features Make Captivating Images
From a variety of microscope images, analytical spectroscopy techniques, and computer graphics, scientists often find that their work is more than just science—the illustrations they create can be astonishing. In 2003, C&EN’s year-end Chemistry Highlights feature included two captivating graphics that when viewed today give one pause to wonder what became of the research that created them.
One of the figures is a schematic drawing depicting the behavior of a self-assembled monolayer of alkanethiol molecules that could be made to reversibly stand or collapse by applying an electrical potential (Science 2003, DOI: 10.1126/science.1078933). At the time, the switchable surfaces, created by serial inventor Robert S. Langer of MIT and coworkers, were thought to be useful for microfluidics, drug delivery, electro-optics, and offset printing. Langer says several of his colleagues have followed up on the technology in novel ways, including making asymmetric nanoparticles.
Joerg Lahann, now a professor at the University of Michigan, Ann Arbor, led the MIT team that created the surface by wetting a gold electrode with an alkanethiol capped by a carboxylate end group. The transition between straight (hydrophilic) and bent (hydrophobic) alkanethiols was triggered by the flip of a switch: Applying a potential to the gold substrate attracts the carboxylate tips down to the surface, hiding the hydrophilic carboxylate groups and exposing the hydrophobic alkyl chains.
“There has been quite a bit of follow-up work from many groups, including my own research group,” Lahann says. “I think it’s fair to say that paper sparked a new direction for active and autonomous materials. The paper has been cited more than 600 times by now, and it is still regularly referenced.” In addition to switchable monolayers, Lahann’s group has developed different approaches to making switchable surfaces, including magnetically switchable particle arrays that change color.
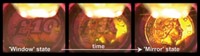
A second eye-catching figure from 2003 stems from research to create solid chiral surfaces, which can be used as enantioselective catalysts or for sensing chiral molecules (Nature 2003, DOI: 10.1038/nature01990). The two images, created in the lab of Jay A. Switzer at Missouri University of Science & Technology, are graphical representations of X-ray diffraction data. The peaks are stereographic projections—so-called pole figures—showing the crystal orientation of enantiomeric copper oxide films electrodeposited on achiral gold substrates. Switzer’s team used right- or left-handed tartrate molecules in the deposition solution as templates to orient the films with right- or left-handed chirality.
Since 2003, Switzer’s group has deposited chiral copper oxide on different substrates and shown that the enantiospecificity can be enhanced by etching the films using chiral etchants (J. Am. Chem. Soc. 2007, DOI: 10.1021/ja073640b). “I am sad to announce that I have not formed a start-up company and made billions of dollars—yet,” Switzer informs C&EN. Switzer’s lab is still working on electrodeposition of metal oxide and metal hydroxide films, with an eye toward developing oxygen-evolving catalysts for splitting water to produce hydrogen (Chem. Mater. 2013, DOI: 10.1021/cm400579k) and to make switches for solid-state memory devices being designed for smartphones and tablet computers (ACS Nano 2013, DOI: 10.1021/nn4038207).






Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter