Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Good, Bad, Ugly
by A. Maureen Rouhi
February 11, 2013
| A version of this story appeared in
Volume 91, Issue 6
Tuberculosis made the news several times last week. The good news is that the group of emerging economies known as BRICS—Brazil, Russia, India, China, and South Africa—has agreed to join forces in fighting the scourge. The bad news is that a new vaccine being developed has failed expectations. The ugly news is that TB drugs for sale in many developing countries are substandard.
C&EN has been covering developments in TB research since the late 1990s, when the disease resurfaced as a public health emergency. Back then, I wrote: “Eradication of tuberculosis will boil down to new drugs and new vaccines, the sorts of things only drug companies can make. Whether enough companies will join the battle is not clear” (C&EN, May 17, 1999, page 52).
More than a decade later, enough drug development groups have joined the battle—including nonprofits, biotech companies, and major pharma companies—for progress to happen. Many countries have joined as well, the latest being the BRICS nations. At a meeting in New Delhi last week, health ministers from the five countries pledged to work together to develop capacity and infrastructure to stop the disease through new drugs, vaccines, and diagnostics. They also resolved to collaborate on clinical trials.
The good news from New Delhi will help solidify the progress in reducing TB deaths around the world. The global mortality rate has decreased by 41% since 1990, according to the World Health Organization. Still, in 2011, 8.7 million people got infected and 1.4 million people died from TB, according to WHO. A major challenge has been the explosion of multi-drug-resistant TB, which cannot be cured by the two most potent first-line TB drugs.
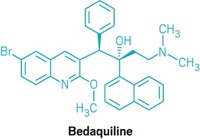
A long-awaited milestone was FDA’s first-ever approval, on Dec. 31, 2012, of a drug developed specifically to treat multi-drug-resistant TB. Sirturo (bedaquiline), discovered by scientists at Johnson & Johnson, is also the first TB drug in 40 years with a new mechanism of action. Instead of attacking the cell wall of the mycobacteria that cause TB, Sirturo inhibits mycobacterial ATP synthase, which the bacteria need to produce energy.
Another long-awaited milestone would have been a new vaccine. Developed at Oxford University, MVA85A was supposed to enhance the protection afforded by the traditional vaccine, called BCG. Last week researchers reported inThe Lancet that MVA85A is no more effective than a placebo in protecting infants who had been vaccinated with BCG at birth. This news is bad; among other setbacks, it might mean loss of funding for further development. But it has a silver lining. Negative results can yield valuable information for future work. And the vaccine may still prove effective for other patient populations.
More than a setback is the finding of a study published last week in the International Journal of Tuberculosis & Lung Disease. Researchers analyzed TB drugs for sale in 17 countries (Angola, Brazil, China, Democratic Republic of Congo, Egypt, Ethiopia, Ghana, India, Kenya, Nigeria, Russia, Rwanda, Thailand, Turkey, Uganda, United Republic of Tanzania, and Zambia) and found that on average, 9% of the drugs did not contain the proper amount of active pharmaceutical ingredient.
This news is really ugly, because substandard drugs will not only fail to cure patients but also promote drug resistance. Writing in the New York Times, Roger Bate, one of the researchers, says: “Some patients will die outright when shoddy medicines fail to cure them. Others will take drugs with too little active ingredient, killing some of their infection’s bacteria but leaving the strongest to multiply. These patients could go on to spread a drug-resistant form of the disease, which is deadlier and vastly more expensive to control.”
Substandard medicines will likely halt the progress made in curbing TB. And just because we are in the U.S. doesn’t mean we cannot be affected. “Since the U.S. is currently facing a shortage of tuberculosis medicines, it will look to sources outside the country,” Bate points out. “It must ensure that these drugs are of sufficient quality.” Amen.
Views expressed on this page are those of the author and not necessarily those of ACS.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter