Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Prescribed Routes: Final Drug Form And Delivery Route Affect Function And Use
by Ann M. Thayer
February 11, 2013
| A version of this story appeared in
Volume 91, Issue 6
A drug’s final form has a big list of medical and commercial implications. It can dictate effectiveness, safety, acceptability, handling, stability, and applicability to diseases. Alternative formulations, such as pediatric doses, can reach new patient groups, revive abandoned compounds, and extend patent life. At the same time, regulators, health care providers, and insurers are pressuring the industry to develop new, differentiated products with clear benefits over old ones.
COVER STORY
Prescribed Routes: Final Drug Form And Delivery Route Affect Function And Use
But medicines aren’t useful if people won’t take them. A recent analysis by the nonprofit New England Healthcare Institute found that 50% of U.S. patients don’t take their medicines as prescribed. Improving compliance could save the health care system up to $290 billion annually, NEHI estimates. To make treatments more attractive, developers need to consider how a formulation can affect the number, type, size, and duration of doses, and a therapy’s taste, odor, and side effects.
Most small-molecule drugs are taken orally, and most biologics are injected. Although patients prefer pills or capsules, these delivery forms may not be feasible depending on a drug’s properties—such as whether it can withstand the stomach—and the target disease, Frost & Sullivan market analyst Debbie Toscano points out.
For example, oral forms tend to act systemically and may not be appropriate for drugs that work better or cause fewer side effects when targeted to a specific part of the body. Inhalation, topical, and ophthalmic delivery methods can target specific conditions. More efficient drug delivery may reduce toxicity and the amount of active ingredient needed.
Improved bioavailability and therapeutic profiles can be achieved in oral drugs through immediate- and controlled-release pill and capsule formulations that dictate when, where, and how fast a drug is released. Manufacturing methods, such as those that can make multilayer pills, allow for delivering more than one drug at a time.
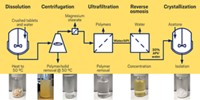
Formulation aids and enhancers are used to alter pharmacokinetics and a drug’s movement through the body. Coatings and capsules can improve drug stability and control dissolution. Producing the final dosage form involves finding the best physical form of a compound, engineering particle sizes or making dispersions, and choosing a delivery route, all in an acceptable size and shape.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter