Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Microscopist Chemists
Methods for imaging samples in reactive environments advance microscopy and materials science
by Mitch Jacoby
January 6, 2014
| A version of this story appeared in
Volume 92, Issue 1

As laboratory analytical instruments go, transmission electron microscopes can be incredibly complex. But the explanation for their broad appeal across much of science is quite simple: Seeing is believing.
Very few lab techniques can provide a view of individual atoms. Delivering that kind of up-close look is transmission electron microscopy’s calling card. But imaging isn’t the only thing TEM offers.
“TEM provides an amazing array of analytical signals that we can use to understand the way materials undergo reaction,” says Pratibha L. Gai, professor of electron microscopy at the University of York, in England. In addition to capturing images with atomic-scale resolution, TEM records diffraction data that can be used to probe details of crystallinity and phase changes in nanoscale solids. And through the interaction of a specimen with the near-speed-of-light electrons that zip through the microscope’s column, TEM also generates X-ray- and electron-energy-loss signals that can be used to elicit details of a material’s elemental composition, chemical bonding, and electronic properties.
What TEM has traditionally not provided is a way to examine materials while they are subjected to the kinds of conditions typically encountered in real-world applications. Judging by the recent bounty of journal papers on this topic, things are changing. Scientists gathered at last month’s Materials Research Society meeting in Boston to discuss the latest advances in TEM methods for analyzing technologically important materials under conditions that not long ago would have been nearly impossible.
Examples include solid catalysts exposed to high pressures of reactive gases at elevated temperatures, and electrodes or other solids in contact with liquids. Those conditions are par for the course in chemical reactors and batteries, respectively. But historically they were off-limits to electron microscopes, which were—and for the most part, still are—designed to operate at high vacuum and room temperature. Flooding a microscope with any gas, especially a reactive one, and heating were sure ways to ruin the instrument.
To bypass those limitations, Gai and longtime collaborator Edward D. Boyes, a professor of physics and electronics at York, designed a microscope column in the 1990s that could tolerate those conditions. The team modified the microscope’s electromagnetic lenses and other components in a way that enables gases to be delivered to and pumped away from a heated specimen. The one-of-a-kind design selectively exposed the specimen region to high temperatures and gas pressures to mimic industrial reaction conditions, but it did so without adversely affecting the microscope’s sensitive electron optics and hence its resolution. Although the prototype instrument provided an unprecedented angstrom-level way to spy on catalysts in their reaction environments, the highly specialized nature of the instrument and experiments meant that for years very few research groups could carry out this type of TEM work.
TEM techniques developed more recently now enable many groups to conduct these types of studies, which aim to capture chemical, structural, and other changes induced in materials by their reaction environments. Rather than comparing the materials before and after they have changed and inferring the evolution process, scientists are now able to understand the reaction mechanisms and dynamics by directly monitoring changes while they occur. Their goal is to use that information to design and make better-performing, longer-lasting, and otherwise improved catalysts, battery electrodes, and other technologically important materials.
For example, when it comes to solid-state catalysts, “structure sensitivity is more often the rule than the exception,” says Stig Helveg, a research scientist at Danish catalyst manufacturer Haldor Topsøe. In these types of catalysts—which drive petroleum and hydrocarbon chemistry, automobile emissions cleanup, and other large-scale processes—catalytically active sites are often subtle, minority surface features such as defects and groups of atoms in low-coordination sites. Detailed understanding of the structure and function of those sites can provide insights into the relationship between catalyst nanostructure and reaction mechanisms, Helveg says. That information “is often the missing link in catalysis,” he adds.
In one study designed to ferret out such details, Helveg teamed up with Alexis T. Bell and other scientists at the University of California, Berkeley, to understand the process by which carbon accumulates on metal-oxide-supported platinum nanoparticles during hydrocarbon transformations. Such particles are widely used industrially as catalysts to transform alkanes via dehydrogenation, isomerization, and other types of reactions. As these reactions proceed, a film of carbon (or coke) grows on the catalyst surface and alters the product distribution undesirably. If left unchecked, the film can deactivate the catalyst.
To probe the carbon film growth process, which is a first step toward controlling it and protecting the catalyst, the team heated MgO-supported Pt nanoparticles to roughly 500 °C in an isobutene atmosphere inside a microscope—conditions that cannot be achieved in traditional TEM instruments. From a time-lapse series of TEM images recorded under those conditions, the team found that carbon accumulates in the form of graphene sheets, which grow from nanoparticle surface irregularities known as steps.
Details of graphene growth depended on particle size. The group observed that for particles with diameters of less than roughly 6 nm, graphene formed nanotubes and in some cases moved from the metal particles to the MgO support. They also observed that particles larger than 6 nm in diameter were subject to complete encapsulation in multiple graphene layers (J. Catal. 2012, DOI: 10.1016/j.jcat.2011.10.008).
In another study based on this type of analysis, which is variably referred to as in situ, operando, or environmental TEM, Helveg and coworkers at the Technical University of Denmark, Lyngby, analyzed the process through which diesel oxidation catalyst particles sinter—coalesce—when exposed to high temperatures in an oxidizing environment. That destructive process decreases surface area, buries active sites, and degrades catalyst performance.
By exposing a model catalyst—oxide-supported Pt nanoparticles—to air at high temperature and analyzing via TEM, the group discerned numerous sintering details. For example, they showed that changes in particle sizes are largely dominated by Ostwald ripening. In that process, large particles grow at the expense of smaller particles via small-to-large particle material transfer. The team also monitored the time-dependent evolution of nanoparticle sizes and particle size distributions. Armed with these microscopy results, the researchers developed a kinetic model that describes subtleties of the sintering process and predicts the catalyst particles’ physical evolution and stability (J. Phys. Chem. C 2012, DOI: 10.1021/jp2098262).

One catalysis puzzle that has drawn considerable attention for more than two decades is the activity of nanoparticulate gold. In bulk form, the precious metal is rather inert, but when prepared as nanosized crystals, gold springs into action.
Details of a commonly studied reaction—low-temperature nanogold-catalyzed oxidation of CO to CO2—still remain something of a mystery. But Seiji Takeda of Osaka University, in Japan, and coworkers have recently applied in situ TEM to the problem and uncovered hints about the way nanogold mediates the oxidation reaction.
The group found that exposing catalytically active ceria-supported gold nanocrystals to a CO/air mixture at room temperature—reaction conditions that historically would have posed problems for TEM instruments—induces structural changes in gold. Compared with nanocrystals in vacuum, nanocrystals exposed to the gas shrink slightly (<1 Å) in one dimension and expand in another (Science 2012, DOI: 10.1126/science.1213194). In contrast, gold foils do not exhibit those changes, the team reported. And in a follow-up study, they showed that those reaction conditions slightly loosen gold nanoparticles, enabling them to move reversibly on ceria in roughly 1-Å steps and perhaps enhancing their reactivity (Nano Lett. 2013, DOI: 10.1021/nl400919c).
In a twist on the heat-accelerated catalysis studied by in situ TEM practitioners, some researchers are devising ways to use TEM to catch light-driven catalysts in action. As with earlier work in this field, that unusual feat requires coming up with a clever TEM column design—in this case, to deliver light to the specimen without ruining the microscope’s performance.
Photocatalysts are becoming increasingly more common in cleaning air and water, generating sustainable energy, and producing solar fuels. Yet numerous questions regarding photocatalyst structure and function remain unanswered. In situ TEM should prove useful for such studies.
One example of the kind of development going on in this relatively new area is the recently reported work of Arizona State University’s Benjamin K. Miller and Peter A. Crozier. The team built a system for illuminating a specimen inside the microscope with visible and ultraviolet light. The setup includes a high-brightness light source with optical filters, a fiber to guide the light to the sample, and a mechanism for precisely aligning the fiber tip (Microsc. Microanal. 2013, DOI: 10.1017/S1431927612014122).
Electrochemistry is another area in which in situ TEM methods are being used to interrogate materials processes with high spatial resolution. As with catalyst studies, the principal challenge in this area is designing a reactor that can go inside the microscope and not degrade its performance, as would have occurred historically. At Pacific Northwest National Laboratory, Chong-min Wang’s solution is to fashion a novel microscale reactor—a functioning battery—that features a single-nanowire electrode that can be probed as it evolves electrochemically.
In one application of this uncommon strategy, Wang, Northwestern University’s Lincoln J. Lauhon, and coworkers, constructed a nanobattery from a single Si nanowire anode, a metallic lithium cathode, and Li2O, a solid electrolyte. Their aim was to monitor changes in silicon as it is lithiated and delithiated during charge-discharge cycles. Swapping commercial Li-ion batteries’ carbon anodes with anodes made from silicon, in principle, could boost battery capacity severalfold. But currently, silicon anodes fail quickly. A detailed understanding of the reaction mechanisms may lead to longer-lived batteries.
By probing the nanobattery with TEM and electron energy-loss spectroscopy, Wang and coworkers observed that lithiation transforms silicon to an amorphous Li-Si state. Then, as the Li:Si ratio reaches 3.75, the amorphous material undergoes a spontaneous transformation to crystalline Li15Si4 (ACS Nano 2013, DOI: 10.1021/nn402349j).

In related work, Wang and coworkers examined another experimental battery system—a sodium-ion battery with a SnO2 anode. Because of sodium’s abundance, such batteries could be less expensive than Li-ion batteries. But once again, the anode fails quickly. TEM analysis shows that inserting and removing sodium causes SnO2 to undergo a phase transformation that leaves the material riddled with pores. The pores increase SnO2’s electrical impedance, which accounts for its poor performance (Nano Lett. 2013, DOI: 10.1021/nl402633n).
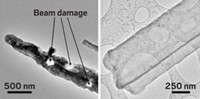
In an effort to mimic real-world operating conditions inside a microscope, Wang and a team of 20 coworkers developed a nanobattery for TEM analysis in which the electrodes are immersed in a standard liquid organic electrolyte (Nano Lett. 2013, DOI: 10.1021/nl403402q). In the older test devices, electrode nanowires make minimal contact with the solid electrolyte and may not be subjected to the full range of electrochemical processes typically encountered in real batteries. As with other developments in this field, this one requires inserting a potentially damaging material into the microscope’s vacuum system, which once would not have been possible.
In situ TEM methods are being applied nowadays to many fields, and practitioners are publishing large numbers of journal papers. Nonetheless, this type of TEM work remains rather specialized, says Michael J. Hepburn, chairman of JEOL UK, part of the JEOL family of instrument companies, which has headquarters in Japan. Conventional TEM is much easier to do today than ever before, he says, but in situ or environmental analyses still require a specialist.
Advertisement
Joerg R. Jinschek agrees. Jinschek is a TEM specialist with microscope maker FEI, based in the Netherlands. He says manufacturers are working to include advanced software and electronic controls to make the instruments easier to operate and experiments simpler to run. Given the power of TEM analysis, those kinds of improvements are sure to make this type of electron microscopy ever more popular and useful.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter