Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Probing Aerosols One By One
Methods reveal the chemistry of individual sea spray and mineral dust aerosol particles
by Celia Henry Arnaud
March 31, 2014
| A version of this story appeared in
Volume 92, Issue 13

Aerosols are the wild cards of climate models. They reflect sunlight back into space, they absorb radiation, and they seed clouds. Climate scientists think that aerosols have a net cooling effect, but the uncertainties in the estimates are so large that no one really knows for sure. Because of the number of variables involved, climate models call for simplifying assumptions, sometimes leading scientists to ignore the chemistry of aerosol particles.
Atmospheric scientists want to know more about the heterogeneity of individual aerosol particle composition in the atmosphere and how to incorporate aerosols most effectively in climate models, said Kimberly A. Prather during a symposium held earlier this month at Pittcon. Prather is a chemistry professor at the University of California, San Diego (UCSD), and director of the Center for Aerosol Impacts on Climate & the Environment (CAICE), a multi-institution Center for Chemical Innovation funded by the National Science Foundation Division of Chemistry. To do that, they need to actually understand the chemistry of aerosols at a deeper level than they currently do—not just the average chemistry, but chemistry at the level of individual particles.
That kind of understanding requires the development of new tools to generate atmospherically representative aerosols in the lab and to measure the composition and reactions of complex aerosols. At the Pittcon symposium, scientists described advances in the quest to create individual aerosol particles and study their chemistry.
COVER STORY
Probing Aerosols One By One
The two most common types of atmospheric aerosol particles are sea spray and dust, which together blanket the globe. For years, modelers have treated sea spray aerosols simplistically, by assuming they are nothing more than sodium chloride in water. But studies by Prather and others are showing that sea spray is much more complicated (Proc. Natl. Acad. Sci. USA 2013, DOI: 10.1073/pnas.1300262110). The problem has been how to replicate that complexity in the lab, where scientists control the aerosol generation process.
Prather and her collaborators exert that control through CAICE’s centerpiece—a wave tank that generates sea spray by using a paddle to create breaking waves. “It took 15 months to generate sea spray that matched ocean aerosols in a low-background environment,” Prather said.
After they succeeded in producing sea spray like that from the ocean, they started tweaking the system to see how the biology of the ocean and other factors affect aerosol chemistry. Instead of just growing algae or bacteria in the tank, Prather and her collaborators “allowed the microorganisms to create conditions and compounds just like they do in the real world,” Prather said. They spiked natural seawater with nutrients and then observed the effects of phytoplankton, bacteria, and viruses on aerosol processes.
The scientists wanted to develop another way to generate aerosols in the lab that didn’t require the use of a 33-meter wave flume. Their new method uses a plunging waterfall that cascades into an enclosed tank for four seconds at a time (Atmos. Meas. Tech. 2013, DOI: 10.5194/amt-6-1085-2013). This method is gentle enough that it allows organisms in the water to continue to grow and thrive.
They then used aerosol time-of-flight mass spectrometry for in situ measurements of the size and chemical composition of individual particles. As the biological system evolved over time, the size distribution and chemical composition of the aerosols changed in a systematic manner that was surprising for such a complex system, Prather said.

Vicki H. Grassian, a chemistry professor at the University of Iowa and codirector of CAICE, also described work on sea spray aerosols. A range of methods is necessary, she noted, because they work at different particle-size scales and are sensitive to the various inorganic, organic, and biological components in aerosols (J. Am. Chem. Soc. 2013, DOI: 10.1021/ja407117x). Grassian’s team examined single particles that varied in size from about 30 nm to 3 μm, and no technique is optimal for the entire size range. In addition to mass spectrometry (MS), she uses electron microscopy and optical spectroscopy to study aerosol particles.
In experiments in which they reacted sea spray particles with nitric acid, which is commonly found in the atmosphere, Grassian and her collaborators observed cation redistribution in the particles in addition to the expected displacement of chloride for nitrate. In unreacted particles, Ca2+ and Mg2+ accumulated at the surface, with Na+ in the core. In reacted particles, the pattern was reversed. In some cases, reacted particles also had a layer of organic material at their surface that wasn’t present in unreacted ones.
This work shows that sea spray aerosols are clearly not just NaCl particles and that treating them as such ignores much of their potential behavior in the atmosphere.
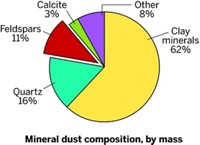
Dust aerosol particles, another common type of atmospheric aerosol, have very different characteristics. The predominant dust aerosols are made of clay, oxide, and carbonate minerals, but their composition can change as a result of processes in the atmosphere, Grassian said.
Grassian used imaging techniques such as scanning electron microscopy, atomic force microscopy (AFM), and Raman microscopy to examine individual dust aerosol particles deposited on a substrate. With SEM, she and her coworkers studied the interaction of gas-phase nitric acid with different components of mineral dust. Calcium carbonate—calcite—particles underwent significant changes, forming a deliquescent layer that picked up water. In contrast, silicon dioxide—quartz—particles didn’t change much, and the reaction was reversible.
Grassian also studied changes in morphology that the particles undergo under different reaction conditions. When oxalic or acetic acid reacted with calcite, the salts were segregated. When quartz particles reacted with humic acids, the organic acids coated the particles, and the particles became more spherical (J. Geophys. Res. Atmos. 2013, DOI: 10.1002/jgrd.50494).
As with sea spray aerosols, dust particles are proving to be more complicated than currently depicted in climate models.
Water is another important player in determining the chemistry of aerosol particles. Allan Bertram, a chemistry professor at the University of British Columbia, Vancouver, described how changes in relative humidity can lead to phase transitions within individual aerosol particles. At high relative humidity, particles consist of a single liquid phase in which the components are dissolved. At low relative humidity, the lack of water causes inorganic salts to effloresce, that is, to crystallize. At intermediate humidity levels, particles have two liquid phases, an organic phase surrounding an aqueous core.

These different phases are important because they affect a particle’s behavior. In the lower atmosphere, uptake of N2O5 depends on the phase: Single-phase aqueous particles can be a sink for various nitric oxides, which are a main component of urban pollution. Reactions of N2O5 lead to a decrease in atmospheric ozone, which is a major air-quality issue. In the upper atmosphere, phase can determine whether a particular particle seeds ice nucleation, which is involved in cloud formation. Single-phase particles are poor ice nuclei. But whether two-phase particles or particles with inorganic cores are good ice nuclei has not been determined yet.
To study how composition influences phase behavior, Bertram and his coworkers mixed 23 organic compounds pairwise with four inorganic salts (ammonium sulfate, ammonium bisulfate, ammonium nitrate, and sodium chloride). They then used various forms of microscopy to determine which particles underwent phase transitions.
In particles that underwent phase changes in response to changes in relative humidity, liquid-liquid phase separation occurred at about 70% relative humidity and efflorescence occurred at about 35% relative humidity. The outer phase was predominantly organic, and the inner phase was inorganic. A “salting-out effect” was also observed as the relative humidity decreased—a decrease in the aqueous solubility of organic molecules as a result of the increasing concentration of salt.
Alexei V. Tivanski, a chemistry professor at the University of Iowa, is also interested in the water-absorption properties of individual particles. He uses scanning transmission X-ray microscopy to determine the hygroscopic properties of particles of different compositions and optical spectroscopy to determine the amount of water in particles. By combining X-ray microscopy with AFM, he can also determine the atomic density of particles, which provides a measure of how porous particles are. At this point, the X-ray measurements are limited to carbon, nitrogen, and oxygen, which restricts the chemical composition of particles that can be studied by this method.
Whereas the other speakers focused on variations in chemical composition, Timothy H. Bertram, a chemistry professor at UCSD, focused on particle-to-particle kinetic variations. He used chemical ionization MS to measure the uptake of N2O5 in sea spray particles in polluted and nonpolluted coastal air plumes off the pier at Scripps Institution of Oceanography in La Jolla, Calif. (Environ. Sci. Technol. 2014, DOI: 10.1021/es4042622). He found that N2O5 uptake in polluted air plumes is influenced most strongly by a particle’s organic fraction, which “can slow down diffusion of N2O5 to the aqueous core of a particle,” he said.
The various single-particle methods are showing that atmospheric aerosols are highly complex, varying from one to another as well as within individual particles. Now that researchers have come such a long way toward their goal of bringing real-world aerosol complexity into the lab, they are poised to open new doors and learn how that complexity translates into reactions that cause significant changes in the atmosphere.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter