Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Where Are They Now? Drug Candidate Updates
by Carmen Drahl , Lisa M. Jarvis
March 31, 2014
| A version of this story appeared in
Volume 92, Issue 13
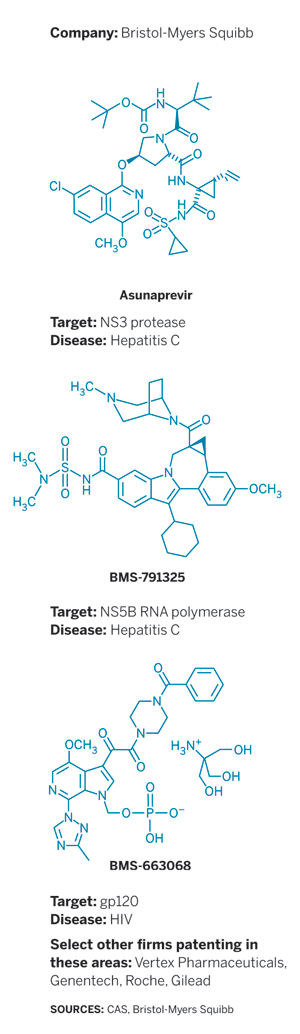
At the ACS national meeting in Dallas, Bristol-Myers Squibb Executive Director of Discovery Chemistry Nicholas A. Meanwell provided updates on three antiviral drug candidates unveiled previously (see structures).
Where Are They Now? Drug Candidate Updates
Meanwell’s talk began with two hepatitis C virus (HCV) drug candidates: the NS3 protease inhibitor asunaprevir (BMS-650032) and the NS5B RNA polymerase inhibitor BMS-791325. He concluded with the HIV attachment inhibitor BMS-663068. All candidates are now in the late-stage clinical studies known as Phase III trials.
If approved, BMS’s HCV compounds enter a highly competitive and rapidly evolving field. For decades, treatment meant a combination of injected interferon and ribavirin, a yearlong regimen that didn’t work for every patient and had brutal side effects.
Beginning in 2011, a series of new drugs worked for a greater proportion of patients and shortened treatment times. Vertex Pharmaceuticals’ Incivek, a protease inhibitor approved as an addition to the regimen in 2011, reached $1 billion in sales in its first year on the market. Earlier this year, Gilead asked FDA to approve a pill that combines Sovaldi, its FDA-approved NS5B polymerase inhibitor, and ledipasvir, its experimental inhibitor of NS5A, a protein that regulates polymerase activity. The duo allows injections to be jettisoned and can cure certain strains of HCV in as few as eight weeks.
BMS is working to keep its HCV portfolio relevant by looking for promising drug combinations. The company is even hunting outside its walls, notably announcing a partnership with Merck & Co. last year. In Dallas, Meanwell focused on BMS’s in-house portfolio.
Meanwell highlighted a midstage clinical trial involving a combination of asunaprevir and daclatasvir, BMS’s experimental NS5A inhibitor. The trial’s results suggested that interferon and ribavirin injections aren’t necessary to cure patients of HCV (New Engl. J. Med. 2012, DOI: 10.1056/nejmoa1104430). A patient is considered cured when viral RNA is no longer detectable in blood plasma.
Encouraged by those results, BMS launched a trial to test a triple-drug cocktail: daclatasvir, asunaprevir, and BMS-791325. Like Gilead’s Sovaldi, BMS-791325 inhibits HCV’s NS5B polymerase. But unlike Sovaldi, BMS’s molecule does not mimic an RNA nucleoside. It acts at an allosteric site of the polymerase rather than at the active site.
In the 66-patient study, the triple-regimen dosed for 12 weeks achieved cure rates of up to 94% (Gastroenterology 2014, DOI: 10.1053/j.gastro.2013.10.057). Multiple Phase III studies of the cocktail are ongoing.
Meanwell then moved on to the experimental HIV therapy BMS-663068. The molecule is a prodrug. Once activated by phosphatase enzymes in the human body, it binds directly to the HIV virus to prevent viral attachment to and entry into immune cells. In a Phase IIb clinical trial, BMS-663068 proved as effective as a combination of Reyataz, BMS’s antiretroviral protease inhibitor, and pharmaceutical company AbbVie’s Norvir, which blocks human cytochrome P450 enzymes to extend Reyataz’s half-life.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter