Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Policy
A Perspective On Reasonable Profits
April 14, 2014
| A version of this story appeared in
Volume 92, Issue 15
Correction
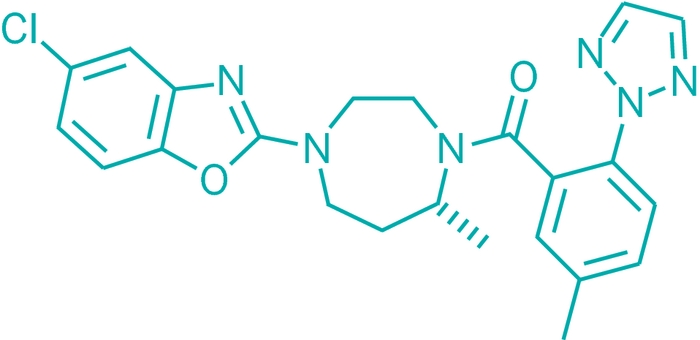
\
March 31, page 26: In a story about new drug candidate disclosures, the structure of suvorexant contains a ketone linkage; it should be an amide, as shown.
“The Year in New Drugs” was quite informative with respect to the variety of drugs the Food & Drug Administration did or did not approve in 2013 (C&EN, Jan. 27, page 10). Big pharma is frequently criticized for the high cost of many of the approved drugs. For example, Gilead Sciences’ hepatitis C drug Sovaldi is mentioned in the article. It is priced at $1,000 a day or $84,000 per year.
However, the article mentions how few drugs will reach the billion-dollar threshold. Development costs and a high frequency of failure of drugs being studied are often cited as the justification for high cost. The breakthrough therapy designation provision of the 2012 FDA Safety & Innovation Act seems a welcome path to more rapid, and perhaps less expensive, drug evaluation and development.
The public reasonably wonders whether the costs are justified. It would have been enlightening if the article included the latest data regarding the actual cost of drug development to put in perspective the balance between development costs and reasonable profits.
Jerome S. Levkovz
New York City



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter