Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Block Copolymers For Lithography
Low-cost alternative to classic methods for making electronic devices exploits molecular self-assembly
by Mitch Jacoby
May 19, 2014
| A version of this story appeared in
Volume 92, Issue 20
With blazing speed and seemingly limitless digital memory, the personal computers of the early 1990s rocketed past those that ranked as state-of-the-art just a couple of years earlier. Within a few years, the new computers were outpaced by even faster ones. And soon the faster ones also became outdated.

For decades, the electronics industry has cranked out one generation after another of ever-faster, smaller, more powerful, and less expensive devices. It has done so through clever device engineering and by cramming greater and greater numbers of increasingly minuscule circuits into smaller spaces. The key enabler has been photolithography. But the conventional form of that technology is about to run into a brick wall.
Block copolymers stand ready to help.
“To continue shrinking the dimensions of electronic devices beyond today’s limits, something different has to happen,” says C. Grant Willson, professor of chemistry and chemical engineering at the University of Texas, Austin.
Historically, lithography innovations have come from physics, Willson adds. To make smaller and smaller features, industry has repeatedly and incrementally reduced the wavelength of the lithography light beam. But that approach is no longer feasible, and making tiny features has become prohibitively expensive. This time the innovations may come from chemistry, specifically block copolymers, he says.
Block copolymers are polymeric molecules in which two or more distinct molecular segments are joined covalently. If the segments are chemically incompatible, the polymer spontaneously adopts phase-separated configurations. Picture a pot of cooked spaghetti—in which each wiggly piece is half red and half blue—arranged such that all the blue segments collect at the bottom of the pot and the reds on top. Depending on conditions, phase separation can lead to various types of ordered structures, including layers, cylinders, and spheres, with features as small as a few nanometers (<10 nm). That feature size is smaller than the size available from today’s standard lithography techniques.
The aim is to capitalize on the smallness and orderliness of those structures to usher in the next round of cost-saving, size-reducing lithography innovations.
That goal is driving researchers in academia, along with their counterparts in the integrated-circuit and magnetic data-storage industries, to investigate all aspects of the self-assembly process. They are using experimental and theoretical methods to evaluate the chemical and physical properties of the polymers, strategies for directed self-assembly to form target patterns, and techniques for implementing the patterns to manufacture electronic devices.
In conventional semiconductor lithography, a light-sensitive polymer known as a photoresist is spun onto a silicon wafer and then imprinted by shining light through a patterned mask. Light exposure leads to chemical differences between the exposed and shaded regions such that one of the regions can then be selectively removed by etching. By depositing metals and other materials in the grooves and on other patterned features, manufacturers construct transistors and other circuit elements in a layer-by-layer fashion.
The advanced lithography method practiced currently is known as immersion lithography and is driven by 193-nm light from argon fluoride lasers. In its basic application, the method can produce patterns in which the center-to-center distance between features can be as small as roughly 80 nm.
As Dow Chemical corporate fellow and polymer specialist Peter Trefonas explains, semiconductor chip makers have developed so-called multiple-patterning techniques for reducing the dimensions of the products of 193-nm lithography. The procedure, which involves a series of iterative deposition and etch steps, allows the critical length to be cut in half repeatedly from roughly 80 nm to 40 nm to 20 nm to 10 nm. But the procedure is painstaking and costly.
“The advantage of block copolymers is that they can help you reach 10 nm or below without needing additional complex lithography steps,” says Phillip D. Hustad, a Dow senior research scientist and directed self-assembly technology leader. But the molecules don’t always line up in orderly and useful patterns with such small features, he stresses. That’s where directed self-assembly comes in.
In the two-tone cooked-spaghetti analogy, segregation leaves the blue layer covering the bottom of the pot and the red layer covering the blue. That horizontal alignment of flat layers generally isn’t useful for lithography, Hustad notes. Instead, researchers have developed procedures to coax the layers to align vertically, such that they stand up perpendicular to the surface. Depending on the alignment procedure, the block-copolymer-coated surface, when viewed from above, could appear to be patterned with alternating parallel stripes. The patterned polymer can then be converted to a patterning mask by selectively removing one of the blocks via etching.
One of the key factors in coaxing the layers to stand up is ensuring that neither polymer segment is preferentially attracted to the surface. In general, that concern can be addressed by first treating the surface with a “neutral” material, one that doesn’t play polymer favorites. An example is a thin film containing a random distribution of the monomers that make up the copolymer, Hustad says.
Getting the layers to stand perpendicular to the surface is only part of the challenge. For lithography applications, researchers want to control the positions of the layers and guide them to form ordered patterns of targeted dimensions. That facet of the self-assembly process can be directed by chemical or physical means.
The chemical method is being investigated widely by industry scientists. Known as chemoepitaxy, it involves decorating the otherwise-neutral surface with carefully positioned microscopic stripes that selectively attract one of the polymer blocks. Segregated polymer domains can also be selectively positioned physically by a method known as graphoepitaxy. That technique calls for prepatterning the surface via lithography with trenches or other features that guide the block copolymers into position.
Several other factors, some of which can be controlled by chemical synthesis, determine the morphological outcome of block copolymer self-assembly. Examples include the number of monomers that polymerize to form the molecular chain and the block-block interaction parameter. Designated by the Greek letter χ, that parameter is “a measure of how much the blocks hate each other,” as Willson puts it. Many of these issues and their interdependence are addressed in detail in a recent review article by Willson, Christopher M. Bates, Michael J. Maher, and coworkers (Macromolecules 2013, DOI: 10.1021/ma401762n).
The most thoroughly studied diblock copolymer for lithography applications is polystyrene-polymethyl methacrylate (PS-PMMA). It’s one of a fairly small number of block copolymers that, at elevated temperature, exhibit balanced interface energetics, meaning neither polymer segment jockeys for position at the air interface.
Research journals are filled with papers describing various applications and methodological developments of directed self-assembly based on PS-PMMA. Last year for example, Michael A. Morris of University College Cork, in Ireland, and coworkers described a graphoepitaxial alignment method that yielded well-ordered patterns consisting of 20-nm PS stripes separated by 19.2-nm PMMA stripes. The team used standard electron-beam lithography to prepattern the surface with guide lines made of hydrogen silsesquioxane (J. Mater. Chem. C 2013, DOI: 10.1039/c2tc00289b).
The property that inhibits PS-PMMA’s polymer blocks from competing for front-row seats at the air interface is related to the polymer’s low χ value. Although a low χ value helps with self-assembled structure stability, it sets a limit on feature size. Specifically, PS-PMMA can’t be used directly to make patterns with a center-to-center distance of less than about 20 nm. That limitation and others have led researchers to examine various other self-assembling block copolymers for their suitability to lithography. Several promising compounds have emerged.

In one example, Willson’s team together with Stephen Sirard and colleagues at Lam Research, Austin, just published a study of two high-χ block copolymers—polystyrene-poly-4-trimethylsilylstyrene (PS-PTMSS) and polystyrene-polymethyltrimethylsilylmethacrylate (PS-PTMSM). They showed that by treating the polymers with protective neutral top coats, the compounds can self-assemble in well-ordered layer patterns (including the one shown on the cover of this issue of C&EN) with center-to-center distances in the 15–22-nm range (Chem. Mater. 2014, DOI: 10.1021/cm403813q).
Another example is polystyrene-polydimethylsiloxane. For several years, PS-PDMS has figured prominently in the nanoscale patterning research programs of Massachusetts Institute of Technology materials science professor Caroline A. Ross and others. In 2007, Ross and Yeon Sik Jung, now at Korea Advanced Institute of Science & Technology, in Daejeon, South Korea, showed that PS-PDMS readily forms well-ordered structures with a low degree of roughness. They also reported that significant differences between the nature of the blocks ensured that one of them can be selectively removed with reactive ion etching—a key requirement for pattern replication (Nano Lett. 2007, DOI: 10.1021/nl070924l).
More recently, Ross and coworkers demonstrated that PS-PDMS self-assembly could guide formation of highly ordered arrays of metal nanowires—Ti, W, Pt, Co, and others—with widths as small as 9 nm. That development may be useful for forming electrical contacts and interconnects in highly integrated nanoscale electronic devices (Nano Lett. 2010, DOI: 10.1021/nl1023518).
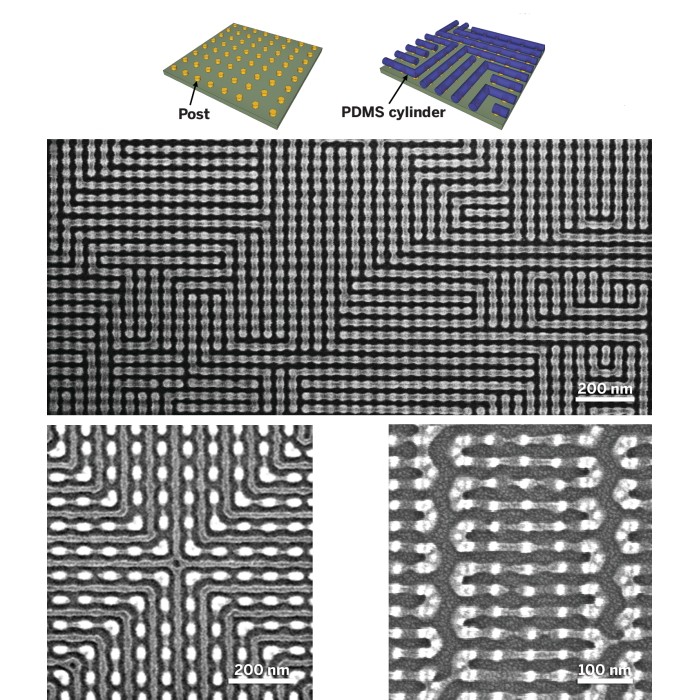
In related work, Ross and MIT’s Karl K Berggren, a professor of electrical engineering, have developed a series of procedures for programming complex self-assembly instructions into a surface. By using electron-beam lithography to prepattern the surface with functionalized nanoscale hydrogen silsesquioxane posts of tailored size and shape, they caused self-assembling PDMS cylinders to adopt unusual and intricate nanoscale patterns. Examples include sharp bends, nested elbows, and meandering and serpentine structures (Nat. Comm. 2014, DOI: 10.1038/ncomms4305).
Now the team is working to extend this type of patterning to form three-dimensional structures (Adv. Mater. 2014, DOI: 10.1002/adma.201400386). Although Ross acknowledges that the idea “is very blue-sky right now,” this type of bulk patterning may eventually lead to a process in which devices are fabricated in a single step rather than in a layer-at-a-time fashion.
Examples of nanoscale patterning from directed self-assembly of block copolymers are numerous. But the method is not yet used to make commercial products. So just how promising is the technology, and how interested is industry?
The decidedly upbeat tone of a paper just published by a large team of IBM researchers makes the computer giant seem interested. The researchers describe how they used this technology to make functioning transistors with nanoscale silicon finlike structures. The team notes that “anticipated” improvements in block copolymers will lead to improved fabrication procedures and better device performance (ACS Nano 2014, DOI: 10.1021/nn501300b).
Meanwhile, at Intel, Senior Fellow Yan Borodovsky says, “This isn’t the first time the microelectronics industry is looking for new patterning technology on the presumption that an extension of a previous technology will not be possible.” As a 30-year veteran, he’s intimately familiar with these kinds of concerns and the solutions that were proposed previously to address them.
“As a technologist, I don’t look for a panacea for all the problems I have.” He says he would rather have a handful of solutions that work adequately than a single cure-all. “Directed self-assembly of block copolymers is very promising for extending today’s advanced lithography methods,” Borodovsky asserts.
It may also enable earlier introduction of other advanced lithography methods currently under development. Although block copolymer technology has been progressing rapidly, “it’s not yet ready for prime time,” Borodovsky says. “So we’ll continue to work on it.”
Ricardo Ruiz is more emphatic. He’s a research manager at HGST, a computer disk drive manufacturer in San Jose, Calif. His firm and others like it want to increase the areal density of magnetic bits—the data-storage elements on computer hard drives—from today’s roughly 700 Gbits per sq in to more than 1.5 Tbits (terabits, trillion bits) per sq in. One promising approach to that challenge is known as bit patterned media, which involves lithographically patterning every magnetic bit.
Given the exacting combination of needs of that application, which are related to detecting magnetic bits in hundreds of thousands of circular tracks on a rapidly spinning disk, Ruiz says, “For now, lithography based on self-assembly of block copolymers is the only serious contender” for meeting that density goal.
Advertisement
Ruiz’s confidence is well-founded. A year ago, members of HGST’s patterned-media group published a study showing how they had used block copolymer directed self-assembly coupled with a double-patterning lithography method to prepare a master template for nanoimprinting patterns with a density of 1 trillion dots per sq in (IEEE Trans. Magn. 2013, 10.1109/tmag.2012.2227303). Since then, the team has boosted the areal density to 1.6 trillion dots per sq in. The template consists of an ordered pattern of raised rectangular pillars measuring roughly 8 × 13 nm.
Making rapid progress in such a technically challenging area requires input from a large number of scientists in many disciplines, Ruiz says. Success depends on the support of chemists, physicists, chemical engineers, electrical and computer engineers, and others. “The work is multidisciplinary and demanding. But the technology keeps improving because these people are dedicated and they love what they do.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter