Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
GABA Receptor Structure Solved
Structural Biology: Crystal structure of human receptor uncovers previously unknown agonist
by Celia Henry Arnaud
June 13, 2014
| A version of this story appeared in
Volume 92, Issue 24

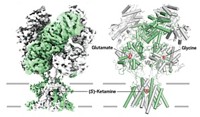
Despite the importance of γ-aminobutyric acid type A (GABAA) receptors as drug targets for a wide range of health conditions, a crystal structure has never been determined—until now. Paul S. Miller and A. Radu Aricescu, structural biologists at the University of Oxford, in England, have obtained the first crystal structure of a human GABAA receptor, at 3-Å resolution (Nature 2014, DOI: 10.1038/nature13293).
Drugs to treat epilepsy, insomnia, anxiety, and panic disorder have been created with this receptor as their target. The receptor is also the target of the general anesthetic propofol. Researchers have long sought structure determination of the receptor to better understand the target and pursue more rational drug design.
GABAA receptors are a family of pentameric chloride ion channels composed of combinations of any of 19 types of subunits. The most common combination is two α subunits, two β subunits, and one additional subunit. The receptor Miller and Aricescu crystallized contains five β3 subunits.
“There is no evidence that a receptor with such composition exists in the brain,” cautions Uwe Rudolph, a professor of psychiatry at Harvard Medical School who studies GABAA receptors and was not involved in the current work. “However, it is a big advance to have a crystal structure of a mammalian—in this case even human—GABAA receptor.”
Miller and Aricescu tested thousands of small molecules for their ability to help crystallize the GABAA receptor. “Benzamidine bound to the neurotransmitter site and stabilized the receptor, allowing us to finally get well-diffracting crystals,” Aricescu says.
Moreover, he and Miller found that benzamidine acts as a receptor agonist. It “promotes opening of the chloride channel and offers a new avenue for the rational design of drugs that modulate the action of GABAA receptors,” he says.
The crystal structure reveals that the β3 pentamer is a cylinder about 110 Å tall that spans the plasma membrane and extends about 65 Å outside it. The receptor’s diameter ranges from 60 to 80 Å.
Miller and Aricescu found that each of its subunits is decorated with three glycans, one of which is deeply buried in the structure and is resistant to enzymatic cleavage. They propose that the buried carbohydrates facilitate communication between parts of the receptor.
Erwin Sigel, a researcher at the Institute of Biochemistry & Molecular Medicine at the University of Bern, in Switzerland, comments that although the analyzed receptor may not occur naturally, its structure could “provide a superior template for homology modeling of GABAA receptors consisting of other subunits. So far, only structures of more distantly related proteins were available.”
“This is a pioneering breakthrough on a very important receptor,” says James M. Cook, a medicinal chemist at the University of Wisconsin, Milwaukee, who designs and synthesizes GABAA receptor agonists. “As scientists learn how this all-β ion channel behaves, the understanding of fully functional systems will not be far behind, at which point drug design for the central nervous system can take a much needed step forward.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter