Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Allylic C–H Oxidation Yields Cyclic Ethers
Palladium-catalyzed C–H activation route uses alcohol nucleophiles for the first time to make cyclic ether drug candidates
by Stu Borman
July 14, 2014
| A version of this story appeared in
Volume 92, Issue 28
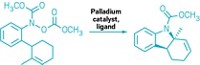
A palladium-catalyzed C–H bond-activation reaction starting with terminal olefins makes it possible to synthesize pharmaceutically important six-membered cyclic ethers with unprecedented generality (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja503322e). Cyclic ethers such as chromans and pyrans are common elements in bioactive small molecules. But reactions used to create cyclic ethers are highly varied, involving different types of starting materials, catalysts, and reaction conditions—potentially making it difficult to decide which synthetic route to take. Using alcohols as nucleophiles to functionalize C–H bonds has not been one of the available options for making cyclic ethers. M. Christina White and coworkers at the University of Illinois, Urbana-Champaign, have now devised a Pd(II)/sulfoxide-catalyzed allylic C–H oxidation reaction that for the first time uses alcohol nucleophiles for that purpose. The reaction has a novel proposed mechanism in which allylic C–H cleavage to form a π-allyl intermediate, alcohol deprotonation, and C–O bond formation all occur at the palladium metal center. White’s team believes the reaction will be useful to chemists seeking to access cyclic ether-based drug candidates.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter