Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Learning An Old Drug’s New Tricks
Researchers seek mechanism for cancer drug bexarotene’s alleged activity against Alzheimer’s
by Lauren K. Wolf
January 20, 2014
| A version of this story appeared in
Volume 92, Issue 3

Finding a new purpose for an old drug is cause for celebration among pharmaceutical firms and patients alike. Using a compound that’s already been tested for one disease to treat another eliminates steps in the drug-vetting process and potentially gets medicine to patients more quickly.
So it’s not surprising that bexarotene, a molecule approved more than a decade ago by the Food & Drug Administration for treating a form of lymphoma, made headlines in early 2012 when it showed promise as a treatment for Alzheimer’s disease. A research team led by Gary E. Landreth of Case Western Reserve University School of Medicine demonstrated that bexarotene could clear amyloid plaques—the clumps of misfolded peptide that are a hallmark of Alzheimer’s—from the brains of lab mice. The drug also appeared to restore the rodents’ memory (Science 2012, DOI: 10.1126/science.1217697).
The initial excitement over bexarotene turned to skepticism a year later, when four research teams reported they couldn’t completely replicate the Case Western Reserve group’s findings (C&EN, June 10, 2013, page 30). Some teams observed cognitive improvements in their bexarotene-treated mice, but some didn’t. And none of the groups saw a decrease in the plaques riddling their rodents’ brains.
In the wake of the confusion, researchers have been taking a closer look at how bexarotene might work—if at all—in nerve cells. Some scientists have collected data that jibe with the mechanism originally proposed by Landreth. Others are proposing a radically different way the drug might work.
Landreth’s original rationale for pitting bexarotene against Alzheimer’s is the compound’s ability to bind to and activate proteins called nuclear receptors. Bexarotene activates a pair of these receptors—one being a retinoid X receptor—which in turn switch on a gene that makes apolipoprotein E (ApoE). When combined with fatty compounds called lipids, ApoE forms spongelike globs thought to soak up and clear toxic compounds from the body. Landreth guessed bexarotene might help wipe amyloid plaques from the brain by increasing ApoE’s production.
When other teams didn’t see the drug clear plaques from their lab animals’ brains, that mechanism was called into question.
Most of today’s Alzheimer’s experts have set aside the notion that amyloid plaques are the toxic culprit responsible for the disease, says Jacques Fantini, a biochemist at Aix-Marseille University, in France. They now believe amyloid oligomers—smaller, water-soluble peptide aggregates—are responsible. A few of the groups that tried to replicate the original Case Western Reserve study, in fact, did see a decrease in this type of soluble amyloid in their mice.
Landreth has also shifted his proposed mechanism’s emphasis slightly, suggesting that bexarotene helps remove amyloid oligomers, rather than amyloid plaques, from the brain.
Looking for an alternative explanation, Fantini recently noticed bexarotene’s structural similarity to cholesterol, another molecule that’s been linked to Alzheimer’s. People with higher levels of “bad” cholesterol, or LDL, are at higher risk of Alzheimer’s than those with normal levels, scientists have shown (JAMA Neurol. 2013, DOI: 10.1001/jamaneurol.2013.5390). Fantini had already been investigating one way cholesterol might contribute to Alzheimer’s: through amyloid pores.
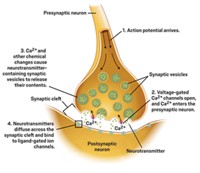
Some experts think amyloid oligomers are toxic because they damage nerve cells by punching holes in them. They hypothesize that amyloid peptides insert into nerve cell membranes, stick together, and make pores, Fantini says. These pores have been observed in artificial membranes in the lab and even in the brain tissue of deceased patients.

Fantini’s research group modeled the pores and their interaction with cholesterol using computer simulations (ACS Chem. Neurosci. 2014, DOI: 10.1021/cn400183w). The scientists found, at least in models, that cholesterol helps stabilize pores made of amyloid.
To confirm these results, the team added amyloid peptide to brain-derived cells. The researchers then measured pore formation by looking at whether calcium surged into the cells—something that happens when neurons are damaged in Alzheimer’s. They observed a flux, but only from cells with sufficient levels of cholesterol. More important, when Fantini’s group added bexarotene, ions stopped rushing into the cells. They took that result as a sign that the compound disrupts cholesterol’s interaction with amyloid and destabilizes the pores.
“My main purpose in carrying out this study was not to prove that bexarotene is a good candidate for treating Alzheimer’s,” Fantini emphasizes. “We are looking for mechanisms.” If valid, though, his pore hypothesis might lead to a rational strategy for discovering Alzheimer’s drugs—ones that inhibit the interaction between cholesterol and amyloid, he says.
According to James S. Nowick, a chemist at the University of California, Irvine, the mechanism put forth by Fantini’s team is intriguing. But he wonders whether bexarotene is exerting its effects by binding to calcium rather than to amyloid. Unlike cholesterol, bexarotene contains a carboxylic acid, which interacts with calcium ions strongly, he points out. “The resemblance between cholesterol and bexarotene may be more superficial than the authors anticipate,” Nowick contends.
Case Western Reserve’s Landreth says the pore idea “is not entirely implausible,” but he sticks by his original mechanism, pointing to a bexarotene study published in October of last year.
The experiment, carried out by Acadia Pharmaceuticals, in San Diego, demonstrated that low doses of bexarotene helped rats with symptoms of Parkinson’s disease, another neurodegenerative condition. The researchers wanted to find molecules that would activate a nuclear receptor called Nurr1 because it maintains the health of dopamine-producing nerve cells—those typically damaged in Parkinson’s disease. When the researchers screened a library of compounds, bexarotene came up as a “hit,” activating Nurr1 in conjunction with a retinoid X receptor.
The Acadia team gave bexarotene to rats with damaged dopamine neurons for 28 days. Not only did the rodents’ motor skills and cognition improve, but a significant number of the animals’ dopamine-producing nerve cells became functional (ACS Chem. Neurosci. 2013, DOI: 10.1021/cn400100f).
The Acadia researchers say these experiments can’t determine whether the activation of nuclear receptors alone accounts for all the positive effects they observed. Fantini takes a similar view: “Bexarotene likely has multiple activities, and one mechanism does not exclude the others.”
While researchers try to better understand a drug that’s stirred up intrigue and consternation in the research community, clinical trials continue. Landreth says a Phase Ib trial of bexarotene now under way in cognitively normal patients should help determine the drug’s mechanism.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter