Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
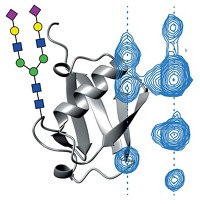
Confirming Structure Of Antibody-Based Drugs
ACS Meeting News: Measuring disulfide pattern via collision-induced unfolding and ion mobility could lead to new tool for quality control
by Celia Henry Arnaud
August 18, 2014
| A version of this story appeared in
Volume 92, Issue 33
Antibody-based drugs are on the rise. Because subtle structural changes can alter their specificity, there’s a need for analytical methods that ensure that antibody-based drugs have the desired structure. A new way to characterize the disulfide bonds that hold antibodies together could help fill that need, reported Brandon T. Ruotolo of the University of Michigan, Ann Arbor. In the method, Ruotolo and his coworkers select an individual charge state of a gas-phase antibody and heat it via gas-phase collisions, which cause it to unfold. They measure the size of the unfolded states with ion mobility. By measuring the unfolding products as a function of the collisional heating, they can track the unfolding trajectory for the intact antibody. The number and size of the unfolded species form a fingerprint that is unique to the original isolated ion. By comparing the gas-phase unfolding for an analyte antibody to that of a reference antibody, the researchers can quickly determine whether they match. Such a method could be used as a quality-control tool to make sure that an antibody drug has the correct structure.
This week’s selections are from the ACS national meeting, which took place on Aug. 10–14 in San Francisco.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter