Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Less Radical Stannylation Mechanism Revealed
ACS Meeting News: Alkyne hydrostannylation long believed to proceed solely via a radical pathway appears to involve a cationic vinylstannane intermediate
by Stephen K. Ritter
August 18, 2014
| A version of this story appeared in
Volume 92, Issue 33
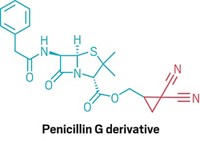
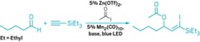
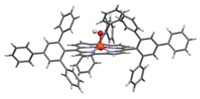
For organic chemists, the free-radical hydrostannylation of alkynes to produce vinylstannanes is a key step in important cross-coupling and other reactions. Every published mechanism has hinged solely on radical intermediates. But that isn’t the whole story, according to Michael G. Organ of York University, in Toronto. Chemists have assumed the reaction goes by a purely radical process because radical initiators such as AIBN or triethylborane are used to trigger the reaction. Organ’s group, in collaboration with Robert D. J. Froese of Dow Chemical, discovered that the reaction is oxygen-dependent. When oxygen is rigorously excluded—a difficult task—the reaction fails completely, Organ said. He suggested that the initially formed vinyl radical is instead oxidized by oxygen to form a cationic vinylstannane intermediate and superoxide. The intermediate undergoes hydride addition from a second stannane molecule, is reduced by the superoxide to generate a stannyl radical, and then reacts with another alkyne molecule to continue the catalytic cycle.
This week’s selections are from the ACS national meeting, which took place on Aug. 10–14 in San Francisco.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter