Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chemists Spruce Up Nickel Aminations
Cross-coupling method uses a small amount of nickel catalyst and standard NHC ligand in a green solvent to prepare gram amounts of aryl amines
by Stephen K. Ritter
September 8, 2014
| A version of this story appeared in
Volume 92, Issue 36
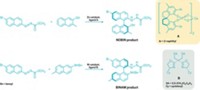
Transition-metal-catalyzed cross-coupling reactions to construct carbon-carbon and carbon-heteroatom bonds are wildly successful. Palladium catalysts have dominated these reactions, but chemists are interested in switching to more abundant, lower-cost metals such as nickel that can operate under greener reaction conditions. To that end, Neil K. Garg and coworkers at UCLA developed a procedure last year for nickel-catalyzed Suzuki-Miyaura C–C couplings that uses a small amount of nickel with a simple ligand in the biobased solvent 2-methyltetrahydrofuran. The UCLA researchers have now developed a complementary method for forming aryl C–N bonds (ACS Catal. 2014, DOI: 10.1021/cs501045v). Their standard procedure calls for using as little as 1 mol % NiCl2(dimethoxyethane) and a commercial N-heterocyclic carbene ligand along with a boronic acid and a base in 2-methyltetrahydrofuran. The team has used the reaction to couple aryl sulfamates and aryl chlorides with morpholine and other cyclic amines to produce gram amounts of new heterocyclic compounds (one shown above). The research received kudos from the journal’s editors, who believe it will be of interest to medicinal chemists focused on identifying drug leads and process chemists looking to develop environmentally friendly routes to synthetic intermediates.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter