Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Nitrogenase Structure Solved With Carbon Monoxide Bound
Substrate analog displaces sulfur from the nitrogen-fixing enzyme’s FeMo cofactor
by Jyllian Kemsley
September 29, 2014
| A version of this story appeared in
Volume 92, Issue 39
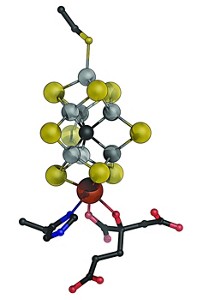
A crystal structure of the enzyme nitrogenase, which some microorganisms use to turn nitrogen into useful ammonia, has at last been captured with a substrate analog bound to its catalytic metal cluster. The structural snapshot reveals that CO can displace a S atom from the enzyme’s FeMo cofactor, a cagelike assembly of Fe, S, C, and Mo, reports a team led by Thomas Spatzal and Douglas C. Rees of Caltech (Science 2014, DOI: 10.1126/science.1256679). Researchers have struggled to unravel how nitrogenase does its N2-reduction chemistry, which could point to better ways to make NH3 than the energy-intensive Haber-Bosch process. CO is isoelectronic with N2 and is a reversible nitrogenase inhibitor. The molecule’s binding to two iron atoms in the cofactor resembles N2 binding on the surface of the iron catalyst used in the Haber-Bosch process, the researchers note.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter