Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Lewis Pair Gets A Handle Just SO On Sulfur Monoxide
Frustrated Lewis pair activation of an N-sulfinylamine provides a rare synthetic source of sulfur monoxide
by Stephen K. Ritter
December 22, 2014
| A version of this story appeared in
Volume 92, Issue 51
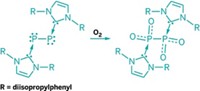
It’s one of those quirks of the periodic table: Molecular oxygen, O2, is stable, ubiquitous in Earth’s atmosphere, and often used as an oxidant in chemical reactions. But sulfur monoxide, SO, just one element removed, is unstable and has a fleeting existence. Chemists have long been searching for ways to stabilize SO to make it useful as a small molecule for organic synthesis, such as complexing it with transition metals, but to little avail. Douglas W. Stephan, Lauren E. Longobardi, and Vanessa Wolter at the University of Toronto have now used frustrated Lewis pairs to tame SO (Angew. Chem. Int. Ed. 2014, DOI: 10.1002/anie.201409969). Frustrated Lewis pairs are acid-base pairs dressed with bulky substituents that prevent them from coming close enough together to completely form a neutral adduct. The resulting reactive species can function like a metal catalyst to activate small molecules such as H2, CO2, CO, and N2O. The Toronto chemists show that the combination of a bulky phosphine and bulky borane can capture N-sulfinylamines to form an unusual seven-membered heterocycle containing six different elements, including an SO unit (shown). In a set of test reactions, the researchers used the new chemical to transfer SO to a phosphine, rhodium complex, or N-heterocyclic carbene, a first step in using SO as a reagent.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter