Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Synthetic Receptor And Peptide Get Cozy
by Stu Borman
March 9, 2015
| A version of this story appeared in
Volume 93, Issue 10
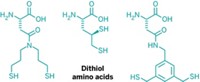
Accomplishing a feat that has “eluded supramolecular chemists for decades,” researchers say they have discovered the first synthetic receptor that binds a peptide sequence in aqueous solution with nanomolar affinity and high specificity, like antibodies do. The receptor-peptide pairing could be useful as an affinity tag for various biological applications. Synthetic receptors are smaller, more stable, more recyclable, and much less expensive than antibodies. But they generally bind peptides with millimolar to micromolar affinities—orders of magnitude less avidly than antibodies. Adam R. Urbach and coworkers at Trinity University, in San Antonio, discovered that the tripeptide Tyr-Leu-Ala binds the donut-shaped synthetic receptor cucurbit[8]uril (Q8) with 7.2-nM affinity (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b00718). The strength of the interaction decreases by three orders of magnitude if Leu and Ala trade places in the tripeptide, exemplifying the receptor’s high specificity. The researchers found the interaction by using a fluorescent indicator to search a broad range of peptide sequences for Q8 binding. Modeling predicts that Tyr and Leu bind snugly inside Q8’s ring and that five hydrogen bonds stabilize the interaction, helping account for its strength.

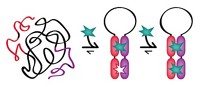

Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter