Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
X-ray Method Unveils How Oil Refinery Catalysts Get Clogged
Metals from crude oil and industrial equipment contaminate pores near the surface of catalyst particles, study shows
by Mitch Jacoby
April 6, 2015
| A version of this story appeared in
Volume 93, Issue 14
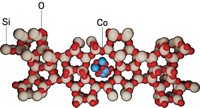
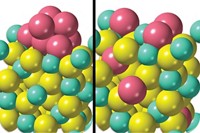
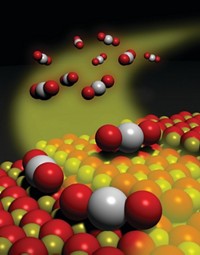
Catalyst particles used to make gasoline industrially become inactive because of metal contamination that clogs pores at the particles’ surface, according to a high-resolution imaging study (Sci. Adv. 2015, DOI: 10.1126/sciadv.1400199). The findings showcase the analytical power of a new X-ray nanotomography method and suggest ways for extending lifetimes of catalysts used globally for petroleum refining. Worldwide, some 450 refineries employ fluid catalytic cracking (FCC) to convert long-chain molecules in crude oil to shorter, more valuable compounds, including ones used for formulating gasoline. During FCC, the catalyst particles—which typically consist of microporous zeolites, clays, and a binder—accumulate metals from crude oil and refinery equipment and undergo irreversible deactivation. By analyzing fresh samples and ones of various ages collected from commercial FCC units, a team led by Bert M. Weckhuysen of Utrecht University, in the Netherlands, found that iron and nickel accumulate at the entrances to “wide” nanometer-sized pores at the particle surfaces. The metals thus block feedstock molecules’ access to the catalytic sites, which are located in narrower interior pores and remain uncontaminated. Coating the zeolites with a macroporous layer may slow deactivation, the team suggests.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter