Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Another Route To Protein Disposal
Proteasome Research: The protein called DJ-1 plays a role in regulating non-ubiquitin-dependent protein disposal
by Celia Henry Arnaud
April 20, 2015
| A version of this story appeared in
Volume 93, Issue 16
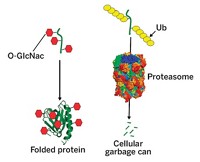
In the primary pathway for discarding proteins, cells tag targeted proteins with ubiquitin and deliver them to the proteasome, the cell’s garbage disposal. But there’s another protein disposal pathway that doesn’t involve ubiquitin and that uses only the central core of the proteasome, a portion called the 20S proteasome. Biologists previously thought this second pathway was a passive, unregulated process. Michal Sharon and coworkers of Israel’s Weizmann Institute of Science now report that the process is regulated at least in part by a Parkinson’s disease-related protein called DJ-1, which is in tissues throughout the human body (Nat. Commun. 2015, DOI: 10.1038/ncomms7609). The researchers found that DJ-1 both inhibits and indirectly activates protein degradation. It inhibits protein degradation by directly binding to the 20S proteasome. But during oxidative stress, DJ-1 activates a protein called Nrf2, which is itself an activator of the 20S proteasome. Sharon and coworkers are now studying where DJ-1 binds the proteasome to figure out the mechanism by which it exerts its effects.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter