Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Molecular Shuttles Get Organized
Nanotechnology: Incorporating a sliding crown ether within a metal-organic framework moves chemists closer to turning molecular shuttles into solid-state nanoscale devices
by Bethany Halford
May 11, 2015
| A version of this story appeared in
Volume 93, Issue 19
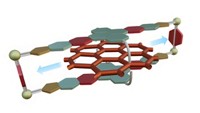
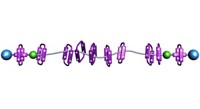
With their ability to switch between two states, the mechanically interlocked molecules known as molecular shuttles hold much promise as components for data storage or nanoscale devices. But organizing these structures so that they might be used practically has been a challenge. Chemists led by Stephen J. Loeb and Robert W. Schurko of the University of Windsor, in Ontario, saw an opportunity to transfer molecular shuttles from the solution state, where their motion tends to be random and incoherent, to a more defined role as a structural component of a solid metal-organic framework (Nat. Chem. 2015, DOI: 10.1038/nchem.2258). They created the molecular shuttle from a dimeric moiety containing a pair of linked benzimidazole units. A crown ether encircling this moiety slides between the benzimidazole groups. Carboxylate groups at the ends of the dimeric moiety coordinate with Zn4O clusters to assemble into an extended metal-organic framework. Solid-state NMR experiments show that the crown ether shuttles can move to and fro throughout the rigid skeleton, establishing that such molecular shuttles can function even when they are densely organized. This achievement, the researchers note, “is a crucial step toward the generation of solid-state nanoscale devices based on mechanically interlocked molecules.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter