Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Molecular Machines
How to make a molecular string of rings
By tethering 2 molecular pumps together, chemists can build polyrotaxanes with exquisite precision
by Bethany Halford
June 11, 2020
| A version of this story appeared in
Volume 98, Issue 23
Making a macroscale version of a polyrotaxane—a string threaded through multiple rings—is a fairly simple task. Young children commonly craft them, for example, by threading yarn through tube-shaped pasta. But making such structures on a molecular scale is a different matter. It’s tough to craft a molecule composed of a precise number of rings on a string when the only tools for assembly are fleeting chemical interactions.
But chemists in Fraser Stoddart’s lab at Northwestern University have done just that. They report a molecular machine that can precisely build polyrotaxanes by using redox chemistry to thread rings onto a polymer chain two at time. The system could be the foundation for information storage systems, with the sequence of rings encoding data, Stoddart says.

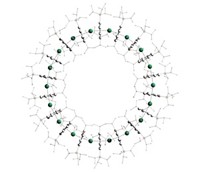
Two molecular pumps help synthesize the polyrotaxane. Each pump is made of a bipyridinium unit sandwiched between a 2,6-dimethylpyridinium end group and an isopropylphenylene group that’s attached to a poly(ethylene glycol) chain, which acts as the string in the polyrotaxane. This setup is key to making the system work, says R. Dean Astumian, a theoretician at the University of Maine, who was part of the research team. The 2,6-dimethylpyridinium creates an electrostatic barrier for molecular rings, and the isopropylphenylene creates a steric barrier. Rearrange those, Astumian says, and the rings will slip off.
In its reduced form, the bipyridinium attracts a ring, a cyclobis(paraquat-p-phenylene), from solution and pulls it over the 2,6-dimethylpyridinium. Subsequent oxidation makes the bipyridinium and the ring charged so that they repel one another. Mild heating pushes the ring over the isopropylphenylene onto the polymer chain. By cycling through this redox process, using chemical reagents or electrochemistry, the chemists are able to add the rings two by two until they then have 10 rings on the polyrotaxane (Science 2020, DOI: 10.1126/science.abb3962). They could add more rings, Stoddart says: “We are only limited by the length of the polymer chain.”
“This method is ingenious and convenient for obtaining new soft materials containing polyrotaxanes,” says Akira Harada, a chemist at Osaka University who studies supramolecular assembly.
Yunyan Qiu, the report’s first author and a postdoctoral scholar in Stoddart’s lab, says the biggest challenge was convincing reviewers that the team had actually made the polyrotaxanes with such precision. “We had to really characterize them and hammer down the number of the rings with concrete evidence,” he says.
“The work shows that noncovalent synthesis has reached the state of precision and control that we know from the much older field of covalent synthesis,” says Roeland Nolte, an expert in molecular nanotechnology at Radboud University. “These elegant studies open completely new directions for exciting research on artificial molecular machines in the future.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter