Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Consumer Safety
After More Than A Decade, FDA Still Won’t Allow New Sunscreens
Even an act of Congress doesn’t move sun filters closer to approval
by Marc S. Reisch
May 18, 2015
| A version of this story appeared in
Volume 93, Issue 20

As summer approaches, bringing time in the sun, people in the U.S. are no closer to benefiting from advanced sunscreen active ingredients than they were a decade ago.
SOURCES: Manufacturers’ Labels, FDA
Download a PDF of this graphic here.

SOURCES: Manufacturers’ Labels, FDA
Download a PDF of this graphic here.
SOURCES: Manufacturers’ Labels, FDA
Download a PDF of this graphic here.

SOURCES: Manufacturers’ Labels, FDA
Download a PDF of this graphic here.
European outdoors lovers, meanwhile, can bask in the sun knowing they have access to new and highly effective sunscreens. These products protect them from ultraviolet (UV) rays that cause sunburn and contribute to wrinkling and increasing rates of skin cancer.
U.S. consumers don’t have access to eight advanced European sun-filtering molecules because the Food & Drug Administration is not convinced they are safe for users. Chemical and cosmetics industry executives counter that people are being denied potentially lifesaving protection. Despite legal efforts to break the stalemate, the wrangling could go on for many more years.
Most sunscreen actives protect users against UV-B rays with wavelengths between 290 and 320 nm, the primary culprits in sunburn. Some also protect against UV-A rays in the 320- to 400-nm range, which can penetrate more deeply into the skin and cause cancer.
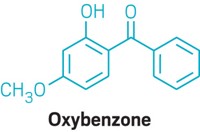
FDA’s current list contains 16 approved sunscreens, just eight of which are regularly used and only two of which offer good UV-A protection. The eight are oxybenzone, avobenzone, octinoxate, octisalate, homosalate, octocrylene, titanium dioxide, and zinc oxide. The UV-A filters are avobenzone and zinc oxide, which is also a good UV-B filter.
Other sunscreens on the FDA list are no longer used because they have an unpleasant feel, irritate skin, or are no longer made. One unused sunscreen, dioxybenzone, turns skin blue on UV exposure.
In a bit of irony, European regulators, who have a reputation for being more cautious about human exposure to chemicals than their U.S. colleagues, allow many more sunscreens. The European Union maintains a list of 27 sunscreen molecules, which, like the FDA-approved sunscreens, are subject to safety testing and maximum dosage restrictions.
The discrepancy is in part because European regulators consider sunscreens to be cosmetics ingredients and not over-the-counter (OTC) drugs, as they are in the U.S. European sunscreen ingredients are not separately listed as drugs on product labels as they are in the U.S. And European formulators are free to combine sunscreens without restriction, unlike their U.S. counterparts, to provide the level of sun protection promised on the bottle.
Four of the eight Europe-only sunscreens, all of which have been submitted to FDA for approval, would provide skincare formulators with more tools against the more dangerous UV-A rays. The others would bolster UV-B protection. Owing to their greater effectiveness, most would also allow formulators to decrease the amount of active ingredient they add and to make sunscreens that feel less goopy.
The new sunscreens could also enliven the U.S. retail sunscreen market, which was valued at $1.5 billion in 2014, according to market research firm Datamonitor. The research firm values the European market at about $2.2 billion.
The Sunscreen Innovation Act, signed into law by President Barack Obama at the end of November 2014, was supposed to help end the U.S. sunscreen drought. The law directed FDA to review applications for the eight European sunscreen molecules: amiloxate, bemotrizinol, bisoctrizole, drometrizole trisiloxane, ecamsule, enzacamene, iscotrizinol, and octyl triazone.
Some of the compounds have been awaiting an FDA response since 2002. Even the Environmental Working Group, an often antichemical advocacy organization, backed passage of the law. Although the group questioned the safety of some of the ingredients that offered mostly UV-B protection, it endorsed the UV-A filters.
The law was a response to an approval process that has origins in a late-1970s decision by FDA to regulate sunscreens as OTC drugs subject to dosage and labeling restrictions. Proposed new molecules are subject to a higher degree of safety scrutiny than if they were regular cosmetics ingredients.
To ease the process, in 2002 FDA established what it called Time and Extent Applications, or TEAs. Sunscreen ingredients with a five-year history of extensive and safe OTC use in another country would be eligible for a fast-track application process that could speed their listing on FDA’s list. The agency promised a yes or no answer in 180 days.
But by 2013, six companies that had submitted TEAs between 2002 and 2009 had heard little from FDA. Frustrated, a group of sunscreen makers joined with cancer prevention organizations, sunscreen formulators, and consultants to involve Congress.
The so-called Public Access to SunScreens (PASS) coalition lobbied Congress and succeeded in getting the Sunscreen Innovation Act passed into law. The law had an effect but not the way its backers had hoped. Even before it passed, FDA began rejecting the TEAs, and by February it had rejected all eight.
The law didn’t tell FDA to approve the eight molecules in limbo, points out cosmetics consultant and chemist David Steinberg. Rather, it only directed the agency to respond to the applications. In each case, FDA asked the makers for more studies that would rule out dangers from chronic exposure, especially for pregnant women and children.
“The FDA,” Steinberg contends, “is bogged down in minutiae because they are overly concerned with sunscreens as drugs.”
Nadim A. Shaath, a chemist who heads Alpha Research & Development, a cosmetics consulting firm, agrees. “If we do not change a fundamental mind-set at the FDA,” he says, “I am afraid that no new UV filters will ever be introduced into the U.S. unless through the lengthy, tedious, expensive, and severely restrictive New Drug Application process.”
In a February posting on FDA’s website, Theresa M. Michele, director of FDA’s Division of Nonprescription Drug Products, explained the agency’s position. “We need more data to decide if these ingredients are, in fact, generally recognized as safe and effective for use in OTC sunscreen products,” she wrote.
Michele acknowledged confusion about why ingredients on the market for years in other countries still can’t be used in the U.S. For FDA, the reasons are clear: Marketing history information submitted through TEAs was limited. “For example,” she wrote, “such information doesn’t tell us anything about the long-term effects from use of the ingredients or how much is absorbed.”
Adding a new wrinkle to the approval process, Michele said FDA was concerned about how daily use of formulations containing sunscreens could affect people. “Because of the widespread daily use of sunscreen products by a broad population, including babies and pregnant women, FDA has proposed data requirements that will allow us to determine that sunscreen ingredients are generally recognized as safe and effective.”
Michele drove those concerns home again in a letter at the end of March responding to a Wall Street Journal editorial critical of FDA’s position on sunscreens. “Americans rely on the FDA to assure that products are safe. Marketing history cannot tell us about associations with long-term effects, such as developing cancer or reproductive problems,” she wrote.
“Of course we have to be safe,” responds Michael Werner, a policy adviser to PASS. But PASS, he notes, is also concerned about increasing cancer rates. The group argues that the eight pending sunscreens are important tools for preventing skin cancer, including the most deadly form, melanoma.
Last summer, the U.S. Surgeon General’s Office noted that 5 million people are treated for skin cancer annually. Melanoma rates skyrocketed 200% between 1973 and 2011. Now, 63,000 new cases of melanoma are diagnosed in the U.S. annually, and nearly 9,000 people die from the disease each year.
“We have a serious problem with skin cancer rates,” Werner says, “but we have FDA holding up all these sunscreen applications.” While other government entities such as the Surgeon General’s Office and the Centers for Disease Control & Prevention encourage the use of sunscreens to prevent cancer, FDA continues to restrict access to more effective sun filters. “The dissonance is really troubling,” he says.
Werner says PASS will “continue to work with Congress and FDA on an appropriate risk model.” But at least two PASS members, the sunscreen actives makers BASF and Ashland, indicate there is a limit to the time and effort they’re willing to put into pushing for new sunscreens in the U.S. market.
For BASF, a major sticking point is that FDA wants preclinical and clinical testing that could take another five years, says Uli Osterwalder, BASF’s sun care scientific adviser. That may take longer than BASF would like.
BASF met with FDA’s Michele shortly after the agency rejected the firm’s TEAs for three sunscreen actives: bemotrizinol, bisoctrizole, and octyl triazone. The first two are broad-spectrum UV-A and UV-B filters. BASF sells them as Tinosorb S and Tinosorb M, respectively. The third is a UV-B filter sold as Uvinul T 150.
“We know that UV-A is potentially carcinogenic, and we have 15 years of marketing experience in over 60 countries with the newer sunscreens to prove their safety,” Osterwalder says. “But FDA won’t go with that thinking. They say times have changed, and they need to look into the filters’ safety more carefully.”
Osterwalder says BASF has offered to collect adverse-effects data covering about 15 years for the three sunscreens. But given FDA’s insistence on seeing more preclinical data, he doesn’t expect the agency to find that information sufficient.
Given BASF’s difficulty with its applications, Osterwalder doesn’t hold out much hope for getting other newer sunscreens on the U.S. market. For instance, the firm has not applied to list diethylamino hydroxylbenzoyl hexyl benzoate, a UV-A filter known as Uvinul A Plus.
Ashland, which licensed bemotrizinol from BASF, says time may be running out. “We can’t go on like this for much longer,” says James Mish, the firm’s group vice president for consumer specialties. He contends that the approval process is just getting too expensive.
After the Sunscreen Innovation Act passed, “we anticipated FDA could still have lingering safety data questions, but we thought those concerns would be reasonable and finite,” Mish says. “We believe the existing data and use studies should be plenty to get a decision made.”
Ashland is willing to conduct additional “reasonable” studies for FDA, Mish says, such as a short-term study based on actual use. However, a long-term study wouldn’t be reasonable, he argues.
Similarly, William Johncock, senior vice president for sun protection at Symrise, is frustrated with FDA’s response. The company first attempted to get amiloxate, a UV-B filter, on FDA’s list, or monograph, in 1981. It switched to the TEA process in 2002, hoping it would expedite approval. Symrise sells amiloxate as Neo Heliopan E1000.

The U.S. decision in the 1970s to designate sunscreens as OTC drugs was a mistake, Johncock contends. European regulators, he explains, consider sunscreens to be cosmetics. They convene scientific committees on an ongoing basis to evaluate sunscreens’ safety and allow new ones to enter the market.
European regulators also react more quickly when questions about a sunscreen arise, Johncock says. For example, aminobenzoic acid, also known as p-aminobenzoic acid (PABA), is still on FDA’s approved list.
European regulators removed aminobenzoic acid in 2009. Critics said the sunscreen provoked allergic reactions in sensitive individuals. Suppliers decided not to defend PABA because of testing costs.
“Are the new data requirements really justified given the long-term safe use of the TEA candidates in many other parts of the world?” Johncock asks. Amiloxate is used worldwide, except for in the U.S., Japan, and Canada, he points out, and was thoroughly evaluated for safety not only in Europe but also by authorities in Australia.
Johncock won’t say whether Symrise will give up on amiloxate in the U.S., but he does acknowledge a dilemma. “FDA’s position puts an economic question on the viability of progressing with our petition, particularly for a product on which our patent ran out a long time ago,” he says.
Merck KGaA developed the chemistry for enzacamene, a UV-B filter, in the 1970s, says Ina Höfgen-Müller, the firm’s global regulatory affairs director. Like Symrise, the firm tried for years to get the filter listed in the U.S, but it has given up for a variety of reasons.
Advertisement
“We’re disappointed because the TEA process was supposed to speed things up,” Höfgen-Müller says. “We actually stopped seeking the monograph listing four years ago when we realized we had no chance of getting this sunscreen listed for the U.S. market,” when suspicions were raised over enzacamene’s potential as an endocrine system disruptor. She maintains that enzacamene is safe.
On top of the FDA impasse, the era of sunscreen development in Europe may be coming to an end, Höfgen-Müller observes. The European Union banned the use of animals to test cosmetic ingredients in 2013, and no in vitro substitutes exist for required reproduction, toxicity, and repeated-dose testing, she says.
So even in Europe, the emphasis in the future will not be so much on new sunscreen molecules. Instead, sunscreen makers will have to work better with the tools they already have, suggests Alexander Kielbassa, business development manager for Merck. In recent years, the firm has developed a range of titanium dioxide filters and a line of silica-encapsulated filters. The technologies reduce the likelihood that the UV filters penetrate skin and cause allergic reactions.
Other sunscreen makers have an interest in the outcome of FDA’s listing process even though they haven’t applied for a new molecule. For example, DSM makes avobenzone, the most widely used organic UV-A sunscreen in the U.S. Jochen Klock, DSM’s global head of sun care marketing, says the firm applied a few years ago for FDA’s approval to allow avobenzone to be used at levels higher than 3%. An answer is pending.
In addition, DSM wants FDA to ease restrictions on combinations of avobenzone with other sunscreens. Lifting them would allow formulators the option to use other sunscreens to keep avobenzone stable on the skin longer.
The firm also has an interest in the approval process because it introduced a UV-B sunscreen called Parsol SLX in Europe seven years ago and would eventually like to sell it in the U.S. Also known as polysilicone 15, the sunscreen has a polymeric silicone backbone “which means it has good film-forming properties and also feels good,” Klock says.
However, DSM executives shouldn’t hold their breath. In the cosmetics industry, “we live and die by making new products all the time,” observes Alpha Research’s Shaath. But with FDA’s approach to sunscreens, he adds, innovation is being stopped dead in its tracks.
Cosmetics industry players question how FDA could stymie public health efforts to reduce the incidence of skin cancer. In the end, they say, it is just easier for FDA to say no: If the agency approves a sunscreen and something goes wrong, it will be blamed for failing to anticipate the problem. Saying no, they say, means FDA will never have to say it is sorry.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter