Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
New Diene Enables Serial Cycloadditions
Organic Synthesis: Chemists create a reagent for tandem reactions to facilitate the synthesis of natural products and imaging agents
by Stephen K. Ritter
May 25, 2015
| A version of this story appeared in
Volume 93, Issue 21
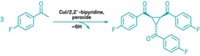
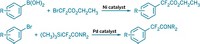
Chemists have created a new class of diene reagents that enable back-to-back cycloaddition reactions all in one operation. The development could help shorten the synthesis of polycyclic natural products and imaging agents while saving time, cutting cost, and reducing waste (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b04091). Stanford University’s Paul A. Wender and colleagues came up with the reagent after pondering how nature often achieves chemical complexity through recursive processes—that is, serially repeating a bond-forming event. They decided to try that approach to amplify the synthetic utility of already-useful Diels-Alder and metal-catalyzed cycloaddition reactions. Using the hard-to-prepare and short-lived tetramethyleneethane (TME) diradical as a model, Wender’s team designed 2,3-dimethylene-4-trimethylsilylbutan-1-ol, known as DMTB. This TME equivalent reacts with a variety of dienophiles in an initial cycloaddition, triggering an elimination step that generates a new diene for a second cycloaddition. The Stanford group has used DMTB to carry out a range of consecutive Diels-Alder and metal-catalyzed cycloadditions. In one example, the researchers used it in the synthesis of a new solvatochromic dye, called 6-DMA, which they expect to be a valuable on-off fluorescence probe for studying biological systems.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter