Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Laser Pulses Drive Chemical Bonding
Chemical Physics: Coherent control of reactions progresses to photoassociation
by Jyllian Kemsley
June 22, 2015
| A version of this story appeared in
Volume 93, Issue 25
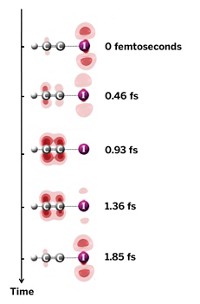
In a step forward for using laser pulses to precisely control which bonds are made and which are broken in a chemical reaction, researchers have used tailored laser light to steer photoassociation of magnesium atoms to form Mg2(Phys. Rev. Lett. 2015, DOI: 10.1103/physrevlett.114.233003). Such coherent control of photodissociation reactions is well established, but it has been difficult to achieve for photoassociation reactions. To produce Mg2, a team led by Zohar Amitay of Technion—Israel Institute of Technology and Christiane P. Koch of Germany’s University of Kassel targeted magnesium atoms at 1,000 K with laser pulses in which the electric field of the pulse oscillates faster as the pulse progresses. The magnesium atoms absorb two photons to form Mg2, which then absorbs a third photon to reach an excited state. The excited-state structure subsequently emits ultraviolet light as it decays to two individual atoms. The structure of the laser pulse affects vibrational transitions in an intermediate state in a way that enhances that UV emission, signaling photoassociation. The next step is to control a reaction that yields stable products, Koch says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter