Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy
Decoding Lithium-ion Conductivity In Solids
Electrochemistry: Understanding ion diffusion in mixed lithium phases may lead to solid electrolytes and safer batteries
by Mitch Jacoby
July 13, 2015
| A version of this story appeared in
Volume 93, Issue 28
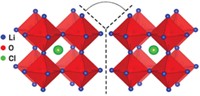
Rechargeable lithium-ion batteries power nearly all of today’s electricity-hungry portable gadgets and tools. Although they boast extreme reliability, the cells’ flammable liquid organic electrolyte solution poses a minute but potentially serious hazard. Nonflammable solid electrolytes would be ideal substitutes for the liquids. But suitable replacements have remained elusive because most solids are weak Li-ion conductors. Mixtures of Li4SiO4 and Li3PO4 are the exception. Compared with the pure components, some mixed compositions exhibit a jump of three orders of magnitude in ionic conductivity. But until now, the phases and their compositions have not been examined in detail, and the conductivity mechanism has not been understood. So a team led by materials chemist M. Saiful Islam of England’s University of Bath applied NMR, electrochemistry, and computational techniques to address those issues, a key step toward making new types of batteries (J. Am. Chem. Soc. DOI: 10.1021/jacs.5b04444). The team found that mixed lithium phases with silicate-to-phosphate ratios of 0.25:0.75 and 0.5:0.5 are among the top solid Li-ion conductors. They also found that Li-ion diffusion proceeds via a concerted hopping motion of interstitial and lattice Li ions through three-dimensional networks, suggesting that increasing lithium disorder may further increase ion conductivity.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter