Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Light-Triggered Chemotherapy
Medicinal Chemistry: Chemists aim to curb side effects of chemotherapy with small molecules that isomerize from an inactive form to an active one in the presence of blue light
by Bethany Halford
July 13, 2015
| A version of this story appeared in
Volume 93, Issue 28
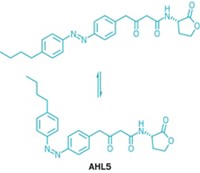
Although many small-molecule drugs target cancer cells’ microtubules, these compounds often work at a patient’s peril. That’s because they don’t distinguish between microtubules of cancer cells and healthy cells, resulting in serious side effects. Chemists have now come up with a strategy to target tumor cells selectively by using compounds that switch from an inactive form to an active conformation when exposed to light (Cell 2015, DOI: 10.1016/j.cell.2015.06.049). Dirk Trauner and Oliver Thorn-Seshold, of Ludwig Maximilian University, in Munich, spearheaded the development of the compounds, known as photostatins, or PSTs. PSTs’ structures are based on that of the natural product combretastatin A-4. The researchers replaced the natural product’s cis C=C with N=N so that it can be photoisomerized easily and reversibly with low-intensity visible light. In the presence of blue light, the PSTs adopt the active cis conformation. In the dark or in the presence of green light, the molecules switch to the trans form. Cell culture experiments show the cis form is 250 times as cytotoxic as the trans form. To treat patients, the researchers envision using light-equipped bandages for cancerous skin lesions and implantable light-emitting diodes for tumors within the body.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter