Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Sulfuranes As Group-Transfer Reagents
Electrophilic Reagents: Expanding on hypervalent iodine, chemists identify imidazolium sulfuranes as a new platform for organic synthesis
by Stephen K. Ritter
July 20, 2015
| A version of this story appeared in
Volume 93, Issue 29

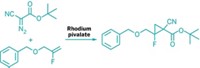
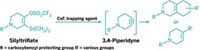
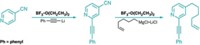
Hypervalent aryl iodine compounds are popular reagents for their ability to transfer trifluoromethyl, alkynyl, cyano, and other electrophilic groups to a variety of nucleophilic organic molecules under mild reaction conditions. With that chemistry in mind, Garazi Talavera, Javier Peña, and Manuel Alcarazo of the Max Planck Institute for Coal Research, in Mülheim, Germany, found it surprising that no one seems to have extended the group-transfer concept to noniodine species. The team envisioned that imidazolium sulfuranes, which have a similar electronic structure to hypervalent iodine species, might be viable alternatives. The researchers designed the new sulfur-based reagents and have now shown that they are useful for cyanation and alkynylation reactions (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b05287). They first halogenated known cyclic thioureas to make hypervalent sulfuranes. Treating the sulfuranes with trimethylsilyl cyanide or silver propiolate led to the desired imidazolium sulfurane cyanates and alkynes, respectively. The team then used the cyano version with amines to make cyanamides and for direct C–H cyanation of aromatics and used the alkynyl version for the alkynylation of thiols, amides, and ketoesters.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter