Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Fluorine Double Take
Fluorinating Reagents: Two groups announce new reagents to more easily introduce fluorine into organic molecules
by Stephen K. Ritter
July 27, 2015
| A version of this story appeared in
Volume 93, Issue 30
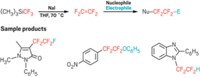
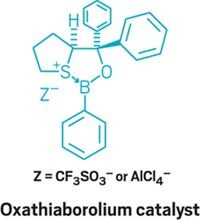
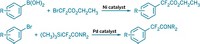
As fluorine chemistry has evolved by leaps and bounds over the past decade, the advances have come hand in hand with the introduction of easier-to-use and more versatile fluorinating reagents. In a new example, Abigail G. Doyle of Princeton University led a team that created 2-pyridinesulfonyl fluoride, or PyFluor, a mild-acting and thermally stable deoxyfluorinating reagent (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b06307). PyFluor replaces alcohol groups with fluorine and has a combination of selectivity, safety, and cost that surpasses that of old standards such as diethylaminosulfur trifluoride (DAST) and complements newcomer sulfur fluoride reagents such as XtalFluor and Fluolead. The researchers show it’s useful in making 18F-labeled compounds for PET imaging. In another example, Qilong Shen, Long Lu, and coworkers at Shanghai Institute of Organic Chemistry have reported N-difluoromethylthiophthalimide as a reagent for incorporating the difluoromethylthio group, –SCF2H, into molecules under mild conditions (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b03170). Chemists who develop pharmaceuticals and pesticides have become enamored with the –SCF3 group because it’s one of the most lipophilic functional groups known. The Shanghai team’s difluoro version offers additional flexibility in tuning the properties of boronic acids, alkynes, indoles, and more.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter