Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Iridium Double Clutch Produces Triborylalkenes
Catalysis: Adding carbon monoxide mid-reaction switches gears for the catalyst, leading to a two-step, one-pot alkyne borylation/boronation
by Stephen K. Ritter
October 12, 2015
| A version of this story appeared in
Volume 93, Issue 40
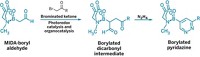
Tetrasubstituted olefins are important structural motifs in drugs and functional materials. Chemists have many ways to make them, including using polyborylated alkenes as intermediates. In exploring alternative routes to the borylated compounds, Oleg V. Ozerov of Texas A&M University and his colleagues previously reported the first example of dehydrogenative borylation of terminal alkynes, in which they used an iridium catalyst with a polydentate SiNN ligand and pinacolborane to make an alkynylboronate. Now they report that they can take the reaction a step further and add two more pinacolborane groups to produce triborylalkenes (Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201507372). The second step is new, Ozerov says, in that diboration of a triple bond is accomplished not by insertion of the alkyne triple bond into a B–B bond, but rather by a second dehydrogenative process involving two B–H bonds. The other thing that is “strange” in this second step, Ozerov adds, is that it requires a different catalyst, but that catalyst can be made directly from the first one. The researchers found they could easily change gears in route by blanketing the reaction with carbon monoxide, which adds CO ligands to the original catalyst. With new triborylalkenes in hand, the team next plans to explore what they can do with them.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter