Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Movers And Shakers
A Conversation With Jack Szostak
The chemical biologist talks about the origins of life.
by Katherine Bourzac
October 19, 2015
| A version of this story appeared in
Volume 93, Issue 41
In the 1980s, Jack W. Szostak made foundational contributions to the fledgling field of genomics. The Harvard University professor of chemical biology was awarded the 2009 Nobel Prize in Physiology or Medicine for his role in the discovery of telomeres, protective caps at the tips of our chromosomes. By the time he received the award, though, he’d long since moved on to perhaps the most fundamental question in biology: How did the first cells emerge from a primordial chemical soup? This June, Szostak and other Nobel Laureates attended the Lindau Nobel Laureate Meeting in Germany. Katherine Bourzac sat down with Szostak at the conference to talk about his work on the origins of life.
What got you excited about working on the chemistry of the first cells?
It’s been a gradual process. After I stopped working on yeast genetics and telomeres, I got really interested in RNA biochemistry. I was interested in this idea that RNA could catalyze its own replication. To make more progress on studying catalytic RNA, or ribozymes, we thought we had to evolve our own. We spent most of the 1990s working on directed evolution of ribozymes. Eventually that got me interested in how this happened naturally. It’s one thing when you have a lab full of fancy equipment and brilliant students—you can evolve molecules to do what you want. But it’s really amazing to think that evolution got started spontaneously. That got me interested in real origin-of-life questions.
What do you think were the conditions under which life emerged?
The scenario I like at the moment is primitive cells growing and dividing in small ponds or shallow lakes in an area that is geothermally active. A modern analog would be something like the geothermal pools in Yellowstone National Park. In a small body of water, you can build up a high concentration of the relevant molecules, as opposed to in the ocean, where everything gets too dilute. On early Earth, you have more heat coming from the ground, which is going to drive groundwater circulation. And circulation is important because, of course, these protocells don’t have any fancy biological machinery to propel themselves or move molecules around.
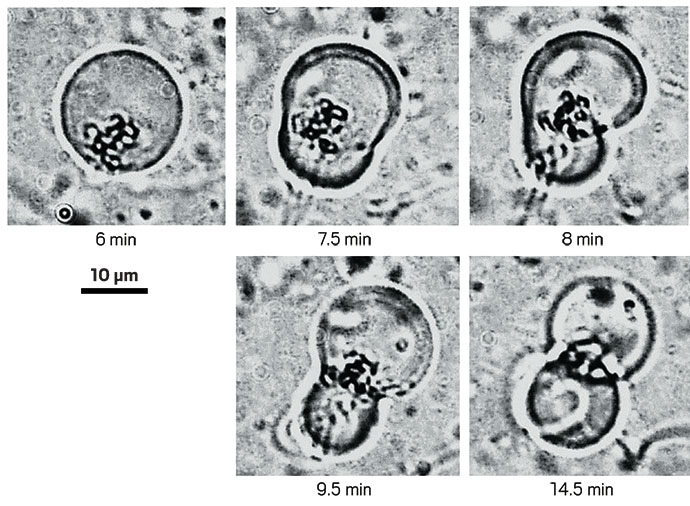
Once there was chemistry to copy RNA strands and make a double helix, then the next step would have been to get those strands apart so that they could get copied again. I imagine primitive cells every now and again getting swept up in a stream of hot water. They’d get heated transiently so that the strands could separate. Quickly they’d move away from the warm current and get cooled. This would have been a way for the environment to drive the cell cycle.
What do you think the first gene was?
One hypothesis that I like quite a lot came from some membrane work performed by a student in the lab. He showed that if you make protocell membranes out of fatty acids but you dope in a little bit of phospholipid, these vesicles grow at the expense of their neighbors that don’t have phospholipid. So the ability to make just a little bit of phospholipid would convey a growth advantage. If there was a ribozyme that could catalyze the formation of phospholipids, it would confer a growth advantage to a protocell.
And of course once you have a ribozyme that’s useful, now there’s a strong selective advantage for replicating it more efficiently. I think that would have led to the evolution of replication machinery, and as the replication machinery got better, the cells could add other functions. This would support the replication of more ribozymes to do more useful things for the cell—a positive feedback situation. If we can see even the beginnings of that in the lab, that would be fantastic.
In your work on protocells, are you trying to replicate what an early cell might have looked like?
If we can create some simple living system, it doesn’t have to be exactly what the first cell was like—in fact, we may never know what the first cell was like. But it’s a starting point. Then we can come up with other ideas that will get us to models of simple cells that may be more like those first cells.
We’re also interested in going the other direction and trying to make cells that are more artificial, that are not anything that could have evolved on early Earth, but that still have the qualities of a living system. I think going in that direction gets at a really interesting problem in chemistry: What is the diversity of chemical systems that can generate life? Is it really limited to the chemistry of modern biology, or is it much broader? The only way to learn that is by trying to build these systems.
Katherine Bourzac is a freelance writer. A longer version of this interview first appeared in ACS Central Science: http://cenm.ag/szostak.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter