Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Agent Could Fight Cancer By Inhibiting Copper Transport In Diseased Cells
Drug Discovery: Small molecule selectively inhibits interactions between proteins in copper trafficking pathway
by Stu Borman
November 12, 2015
| A version of this story appeared in
Volume 93, Issue 45
Normal cells shuttle around copper ions to keep key processes, such as energy production in their mitochondria, running smoothly. But cancer cells also use copper trafficking to grow and spread.
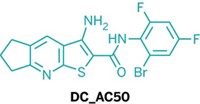
Jing Chen of Emory University School of Medicine, Hualiang Jiang of Shanghai Institute of Materia Medica, Chuan He of the University of Chicago, and coworkers have now developed a selective way of blocking copper transport in cancer cells (Nat. Chem. 2015, DOI: 10.1038/nchem.2381). By screening a database of 200,000 druglike small molecules, the researchers discovered a promising compound, DC_AC50, for cancer treatment. They zeroed in on the compound by testing how well database hits inhibited a protein-protein interaction leading to copper transport and reduced proliferation of cancer cells.
Scientists had already found a molecule, tetrathiomolybdate, that interferes with copper trafficking and have tested it in clinical trials against cancer. But tetrathiomolybdate is a copper chelator: It inhibits copper transport in cells by nonselectively sequestering copper ions. Sometimes, the chelator snags too much copper, inhibiting essential copper-based processes in normal cells and causing side effects.
In contrast, DC_AC50 works by inhibiting interactions between proteins in the copper trafficking pathway: It prevents chaperone proteins, called Atox1 and CCS, from passing copper ions to enzymes that use them to run vital cellular processes. Cancer cells are heavy users of Atox1 and CCS, so DC_AC50 affects cancer cells selectively.
The team has licensed DC_AC50 to Suring Therapeutics, in Suzhou, China, for drug development.
Thomas O’Halloran of Northwestern University, who has studied tetrathiomolybdate, comments that “the challenge in drug design is hitting one of these copper-dependent processes without messing with housekeeping functions that normal cells depend upon. DC_AC50 appears to block the function of copper metallochaperone proteins without interacting directly with their cargo, copper ions. As the first member of a new class of inhibitors, it provides a new way to interrogate the physiology of copper trafficking disorders and possibly intervene.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter