Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Controlling Reaction Selectivity Via Molecular Dynamics
Reaction Mechanisms: When one transition state leads to two products, controlling timing and molecular motion allows for product selection
by Jyllian Kemsley
November 23, 2015
| A version of this story appeared in
Volume 93, Issue 46
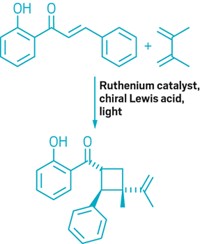
The traditional way to produce only one out of several possible products of a reaction is to stabilize the transition state that leads to that desired compound. But in some reactions a single transition state leads to multiple products, requiring a different approach to selectivity. Such is the case with [1,2]- versus [2,3]-sigmatropic rearrangements. Working with an ammonium ylide containing an enolate group (Y, shown), Bibaswan Biswas and Daniel A. Singleton of Texas A&M University have demonstrated that they can select for either the [1,2] or [2,3] rearrangement product by choosing reaction conditions geared toward promoting particular molecular dynamics paths (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b08635). Alkali-metal bases that do not hydrogen bond to the ylide allow for early formation of the transition state with dynamic motion that favors fragmentation and [1,2] rearrangement. In contrast, methanol or protonated 1,8-diazabicycloundec-7-ene does form a hydrogen bond to the enolate oxygen, increasing the reaction barrier and promoting later formation of the transition state with dynamic motion that favors [2,3] rearrangement.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter