Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Graphene’s Insolubility Drives Materials Synthesis
Method exploits pristine graphene’s knack for stabilizing interface between immiscible liquids
by Mitch Jacoby
February 2, 2015
| A version of this story appeared in
Volume 93, Issue 5
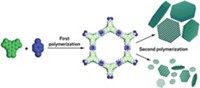
Pristine graphene’s insolubility in both water and common organic solvents limits its use in applications. Researchers often bypass that problem by using oxidized and other chemically altered forms of graphene. But altering graphene generally degrades its properties. So rather than working around the insolubility, University of Connecticut polymer chemists Steven J. Woltornist and Douglas H. Adamson and coworkers exploited it to devise a simple, low-cost method for making new kinds of materials. The team used graphene as a surfactant to stabilize water-in-oil emulsions, capitalizing on its attraction to the interface between water and organic phases. They used the emulsions as templates for synthesizing graphene/polymer foams (Macromolecules 2015, DOI: 10.1021/ma5024236). The composites are lightweight, strong, and electrically conductive, making them candidates for use as construction materials, supercapacitor electrodes, and catalyst supports. The team explains that when graphene is added to a mixture of immiscible liquids such as water and styrene or other monomers, graphene sheets become trapped and spread along the interface, lowering the interfacial surface energy. The sheets stabilize water-monomer emulsions by forming a thin skin around the water droplets. Gentle heating polymerizes the monomer and removes the water, forming rigid foams.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter