Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Scientists Observe Elusive Radical Involved In Combustion And Aerosol Chemistry
Oxidation: Measurements of hydroperoxyalkyl radical may improve engines, atmospheric models
by Jyllian Kemsley
February 6, 2015
| A version of this story appeared in
Volume 93, Issue 6

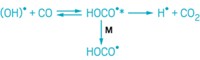
A highly reactive hydroperoxyalkyl radical species has been directly studied for the first time. Rate constants for its formation and for its reaction with oxygen, measured by researchers at Sandia National Laboratories, will help improve models of atmospheric and combustion chemistry (Science 2015, DOI: 10.1126/science.aaa1495).
Researchers believe that hydroperoxyalkyl radical (QOOH) compounds play a key role in chain reactions that generate highly oxygenated molecules, such as those that condense into aerosols in the atmosphere.
QOOH species and their reactions are also key for combustion engines. In spark ignition engines, they can lead to engine knock, so people want to understand the species to control that potentially destructive effect, says Craig A. Taatjes, manager of Sandia’s combustion chemistry department. In contrast, in new compression ignition engines, QOOH species are key to starting and sustaining combustion. Engineers are developing these engines to reduce emissions and increase efficiency.
“These species keep coming up in mechanisms of hydrocarbon oxidation and combustion,” but understanding and modeling the chemistry has been hampered by a dearth of experimental data, says Joseph S. Francisco, dean of the College of Arts & Sciences at the University of Nebraska, Lincoln.
Researchers had previously studied QOOH species indirectly, by tracking their reaction products. “This is the first time we’ve been able to make one stable enough that we could detect it directly and watch the QOOH itself,” Taatjes says.
The Sandia team prepared the radical species by oxidizing 1,3-cycloheptadiene to obtain 2-hydroperoxy-4,6-cycloheptadienyl. Resonance of the cycloheptadienyl ring stabilizes the radical, located on a ring carbon, keeping it around long enough to study.
The scientists characterized the radical and its kinetics using a photoionization mass spectrometer that works with tunable ultraviolet light from the Lawrence Berkeley National Laboratory Advanced Light Source synchrotron. Computational modeling provided insight into the radical’s reaction mechanisms.
“The choice of radicals is ingenious,” says Geoff Tyndall, a senior scientist at the National Center for Atmospheric Research. “The study will certainly help us to probe these systems more deeply and to better understand their complexity.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter