Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chemists Couple Nucleophiles From Two Name Reactions
Organic Synthesis: New reaction is first to marry Suzuki and Hiyama reagents selectively
by Stu Borman
February 12, 2015
| A version of this story appeared in
Volume 93, Issue 7

Researchers have developed a cross-coupling reaction that yields paired-ring compounds by marrying the reactive starting materials from two well-known C–C bond-forming reactions.
Arylsilanes and arylboronic acids are two nucleophilic reagents widely used in “name” reactions to form cross-coupling products with other compounds. But they have never before been convinced to walk down the aisle together. The new reaction fills a niche in chemists’ C–C bond-forming arsenal, easing the way to products that are difficult or impossible to make with existing cross-coupling reactions.
Transition-metal-catalyzed C–C bond-forming reactions are among the most widely used transformations in organic synthesis. In name reactions such as Suzuki and Hiyama coupling, an organic electrophile such as an alkyl or aryl halide combines with an organometallic nucleophile to form a cross-coupling product.
A few cross-coupling reactions that combine two electrophilic reagents or two nucleophilic compounds have been developed previously, but they are much more rare. A common problem with these reactions is strong competition from undesired homocoupling—addition of a compound to itself, instead of to its reaction partner—making it difficult to achieve high selectivity for desired products.
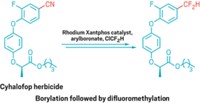
Now, Yanghui Zhang and coworkers at Tongji University, in Shanghai, have developed a reaction that cross-couples two nucleophilic compounds highly selectively (Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201412288). It combines aryl trialkoxysilanes, Hiyama reagents, with aryl boronic acids, Suzuki reagents, to form predominantly cross-coupled products. Palladium(II) with a commercially available 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl (BINAP) ligand catalyzes the reaction through a mechanism that suppresses homocoupling.
Jin-Quan Yu of Scripps Research Institute California, an expert in C–C bond formation, comments that the new reaction successfully addresses the major hurdle of a preference for homocoupling in coupling reactions of two nucleophiles. “It could prove handy in scenarios where Suzuki and Hiyama coupling cannot be used,” he says. For example, “Suzuki cross-coupling is often not compatible with boronic acid reagents containing a bromine or iodine substituent, but with the new reaction that may not be a problem.” If the reaction can be extended to coupling alkyl reagents, “it could become a broadly useful method for making carbon-carbon bonds.”
Indeed, Zhang confirms that “extending the protocol to other substrates, including alkylboronic acids and alkylsilanes,” is under way in his lab. He says he and his coworkers also hope to extend it to the synthesis of chiral biphenyl compounds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter