Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Aromaticity By Any Other Name
C&EN Online Exclusive: Chemists discuss the merits of the unbridled use of the concept of aromaticity
by Stephen K. Ritter
February 22, 2015
| A version of this story appeared in
Volume 93, Issue 8
In the January/February issue of the magazine American Scientist, Chemistry Nobel Laureate Roald Hoffmann took exception to scientists applying the concept of aromaticity beyond organic molecules to inorganic planar and cluster molecules. Here, C&EN presents a response from Alexander I. Boldyrev and Lai-Sheng Wang, who pioneered that trend, along with a rebuttal from Hoffmann. C&EN also asked several chemists who spend most of their time working with and thinking about chemical bonding and aromaticity to weigh in. Add your thoughts about the use or misuse of aromaticity in the comments section below.
Should Aromaticity Be Reserved For Only Benzene And Its Derivatives?
Alexander I. Boldyrev and Lai-Sheng Wang

Alexander I. Boldyrev, Utah State University, studies theoretical and computational chemistry of new compounds and is coorganizer of the International Conference on Chemical Bonding.
Lai-Sheng Wang, Brown University, uses photoelectron spectroscopy to probe the chemical bonding and electronic structures of gas-phase metal, metal oxide, and nonmetal clusters and solution-phase anions.
We recently read a perspective article on aromaticity by Roald Hoffmann, published in American Scientist. In the article, Hoffmann criticized extension of the aromaticity concept to noncarbon systems or transient chemical species and suggested that chemists should stay within the bounds of August Kekulé’s original ideas about aromaticity put forth 150 years ago. We are among those scientists who have extensively used aromaticity and antiaromaticity concepts to describe chemical bonding in many different types of chemical species beyond organic chemistry. Hoffmann specifically criticized our proposal of aromaticity in the square-planar Al42– species as “hype.” We feel compelled to respond to his criticism.
Hoffmann put forward two stringent criteria for molecules to be considered aromatic: Aromatic molecules should not be reactive, and they should be bench-stable or bottleable. Indeed, 150 years ago, chemistry was “bottled” chemistry. Chemists were not aware at that time about isolated cations, isolated anions, radicals, and clusters. Yet these are species chemists encounter and study routinely today on Earth and beyond. Many of these species are reactive and one certainly cannot catch a bottle of them. Does it mean they cannot be aromatic?

About 15 years ago, we started to work on the structure and bonding of size-selected clusters made in molecular beams. We have tried to develop chemical bonding models to understand the stabilities of these new chemical species. We quickly discovered that the bonds in these clusters cannot be described by conventional two-center, two-electron (2c-2e) bonds without using resonance theory.
When we started out, chemists had already used aromaticity to understand the stability of exotic nonbenzene organic molecules, such as the cyclopropenyl cation cyclopropenyl cation (C3H3+). We have found striking similarities in bonding between many of our cluster systems and organic systems and have embraced the aromaticity/antiaromaticity concepts, including σ-aromaticity and double aromaticity (σ and π). Indeed, the umbrella of aromaticity has been extended to shelter many chemical species, including ionic species, radicals, molecules in excited states, atomic clusters, nanoparticles, two-dimensional materials, new compounds that exist only under high pressure, and many others.
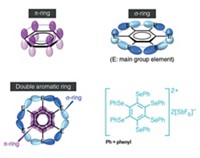
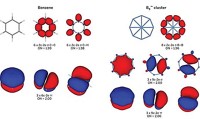
Of course, these new chemical species were not known in Kekulé’s day, but they are common in chemistry today and should be described by the same modern chemical bonding models. Aromaticity is a model that we have found to unify the concept of describing bonding in many atomic clusters, well beyond Al42–. For example, consider the structure and bonding in the B9– cluster, a molecule that has been produced in cluster beams and has had its photoelectron spectra measured, though it is not yet bottled.
Computational global minimum searches show B9– has a beautiful wheel structure, a conclusion confirmed by comparing calculated with measured photoelectron spectra. Why does B9– adopt this structure? Its bonding is intractable using classical localized electron pair models. But its bonding and stability can be easily understood using the concept of double aromaticity, meaning that the compound exhibits σ- and π-aromaticity together.
In B9–, there are 28 valence electrons. Sixteen of them are used to form eight 2c-2e σ bonds between peripheral boron atoms. The remaining 12 electrons are equally split between σ and π systems, but they cannot be localized into 2c-2e bonds. The delocalized π-electron densities have the same shape as the π-electron densities of benzene, hence B9– is π-aromatic. However, if we look at the delocalized σ electrons, they have exactly the same pattern as the π electrons, except there is no nodal plane in the σ bonds. If one accepts π-aromaticity in this cluster, one must accept its σ-aromaticity.
This simple bonding picture allows us to understand why B9– has equal bonds, a ring current similar to benzene, a very large energy gap between its frontier molecular orbitals, and a high first electron detachment energy––all characteristics of aromaticity. Although the chemical reactivity of B9– has not been investigated, it is expected to be less reactive than neighboring nonaromatic boron clusters. In fact, the stability of boron cluster cations toward dissociation has been studied, and B13+ has substantially higher stability than the others. Chemical bonding analyses reveal that B13+ is also doubly aromatic, consistent with its high stability. Indeed, we have shown that the idea of aromaticity is extremely useful in understanding the structure, chemical bonding, and stability of boron clusters.
Hoffmann professed in his essay that because no one can bottle Al42– it should not be considered aromatic. It’s true that since our publication nobody has made a solid-state compound containing Al42–. However, synthetic chemists may now take the challenge to make such compounds, because Hoffmann, being a Cornell man, has promised “a good bottle of New York state Riesling” for the first chemist to make it. More important, our concept of aromaticity in Al42– has been verified by analogous compounds that have been bottled.
For example, K2([(mesityl)2C6H3)]Ga)3, which contains an contains an aromatic triangular Ga3 core, was synthesized even before our publication on Al42–. A subsequently prepared compound containing a square-planar aromatic Ga42– coreis even more closely related to Al42–. Finally, the Na3Hg2 amalgam, whose structure was established in 1954, contains square-planar Hg46– clusters, which are valence isoelectronic to Al42–. both have the same molecular orbital pattern. These bottled compounds provide ample proof for the validity of the bonding description in Al42–. Hoffmann can send his bottles of Riesling to those chemists.
The square-planar Al42– indeed fulfills all the criteria for aromaticity: Hückel’s 4n + 2 (n = 0) π electrons, bond equalization, magnetic shielding, and even high resonance stabilization energy. As the saying goes, “If it walks like a duck, quacks like a duck, and looks like a duck, it must be a duck!”

We do not see any “hype” in the characterization of Al42– as being aromatic. Furthermore, when two more π electrons are added to form Al44–, it is promptly distorted into a rectangular shape analogous to cyclobutadiene, just as the Hückel rule predicts for an antiaromatic molecule. As Hoffmann pointed out, one reason the concept of aromaticity will survive “comes from its inherently chemical and changeable nature.” We would add that the beauty and power of the aromaticity concept lies in its generality—that it can help us understand the chemical bonding of so many different types of chemical species, bottleable or not.
Response To "Should Aromaticity Be Reserved For Only Benzene And Its Derivatives?"
Roald Hoffmann
Roald Hoffmann, Cornell University, is a 1981 Nobel Laureate in Chemistry, recognized for his theories concerning the mechanisms of chemical reactions.
I love molecules, whether they are bench-stable, bottleable, or not. What I react against is hype, the exaggerated attribution of importance to molecules on the basis of their possessing some special characteristic. In the present context, hype takes the guise of a kind of hanging on to the coattails of an idea of something special, something particularly good or virtuous—namely aromaticity. That’s what I feel when I see Al42– or C6 (benzene denuded of its hydrogens) or PtZnH5– touted as aromatic or doubly aromatic.
There is no question these molecules exist and can be studied in a matrix or a molecular beam. AndAlexander I. Boldyrev and Lai-Sheng Wang do point to isoelectronic entities that exist in a crystal, which I missed. I suggest it suffices to point out that Al42– or C6 or PtZnH5– are stabilized, to single out the electronic mechanism of their stabilization, and to study them experimentally.
But to label them as aromatic, with the 150-year-old history of thermodynamic stability, kinetic persistence, and chemical reactivity associated with that concept, as well as the 20th-century correlates we’ve added, is to me—and I think to many—a stretch. To label Al42– or C6 or PtZnH5– or similar metastable molecular entities as doubly aromatic seems ludicrous, a double stretch.
To mention how these compounds possess ring currents, bond equalization, and other criteria of aromaticity without doing the simplest and theoretically easy-to-do test of kinetic persistence (the calculation of a barrier to dimerization of a neutral derivative, or its reaction with typical ambient molecules) is further evidence that the people who study these molecules are mainly reaching after praise by association, or are just invoking catchwords.
It’s possible to recognize stability, even ubiquity, without hype. Take H3+, a lovely equilateral triangle of a molecule, available in vast quantities in interstellar space. The molecule has an energy that is 422 kJ/mol, more stable than H2 + H+. Of course, it has two electrons in a system of three orbitals, and that is the source of its stability and geometry. What is to be gained by calling H3+ singly aromatic? It’s good to see the isomorphism to the electronic structure of the cyclopropenyl cation. I love that. But H3+, as common as it is in space, is very reactive.
I think it suffices to call H3+ stable, to make that connection, so vivifying to chemistry, in recognizing the symmetry-conditioned orbital mechanism of its stability. To term H3+ aromatic, without a word on just how reactive it is, I would say is excess.
That is how I feel about the claims Boldyrev, Wang, and others have made. The B9– wheel they have studied is absolutely lovely. It’s intriguing to see that it has a nice closed-shell electron count in both its σ and π systems. But to label this beautiful boron wheel doubly aromatic, to me, is hype.
Frank A. Weinhold
Frank A. Weinhold, University of Wisconsin, Madison, developed the natural bond orbital analysis method to calculate the distribution of electron density and to spot bonds in molecules.
I admire the work and respect the opinions of all three of these chemists—Roald Hoffmann, Alexander I. Boldyrev, and Lai-Sheng Wang––so I may only add confusion by expressing significant agreement as well as some disagreement with points made on both sides. Theoretical discussions of aromaticity often lead to seemingly contradictory conclusions, depending on how one is choosing to understand the term.
In a sense, their apparent dispute may be largely a matter of how much creative ambiguity ought to be allowed to a time-honored concept that remains in popular usage despite having an intrinsically ill-defined or multiply defined character. Contrary to the apparent linguistic associations with a “smell test,” the original laboratory definition of aromaticity referred specifically to the characteristic property of benzenoid species to resist bromination reactions that are common to ethylene and other simple alkenes. This unusual property thereby came to be associated with the rock-star status of benzene in the spectacular 19th-century growth of chemical theory and the chemical industry.
Advertisement
It is remarkable that August Kekulé’s prescient 1872 depiction of dual cyclohexatriene bonding patterns––resonance structures in Linus Pauling’s language––accurately captures what most theoreticians would still agree is the essence of benzene’s distinctive electronic character. However, subsequent studies led to a time-dependent array of structural, energetic, and magnetic criteria, as noted by Paul von Ragué Schleyer and quoted by Hoffmann, which tended to agree for benzene but not necessarily for other species that might, by one criterion or another, be considered aromatic.
Such a multiplicity of criteria and possible meanings for aromaticity, including numerological 4n + 2 associations, may invite broader applicability of the concept at the price of reduced specificity and clarity in communication. Whether such a point of diminishing returns has been passed in a particular case would be a matter of scientific taste.
Whatever is said should not detract from the beautiful chemistry that Boldyrev, Wang, and their colleagues have uncovered, or the earnest efforts of all concerned to somehow incorporate these amazing species into broader understanding of the phenomenon of chemical bonding.
Gernot Frenking
Gernot Frenking, Philipps University, Marburg, Germany, is a computational chemist who uses quantum chemical methods to study molecules with unusual bonding situations.
I share Roald Hoffmann’s sentiment that there has been an inflation of variants of aromaticity in the past (Y-aromaticity for noncyclic compounds, three-dimensional aromaticity, homoaromaticity, σ-aromaticity, and numerous others). There was a time when I was wondering if there were molecules left that do not exhibit some kind of aromaticity.
However, in the present case, I do not share Hoffmann’s criticism of the notion of σ-aromaticity. His comments reflect the view of a synthetic organic chemist who considers the value of a molecule by whether it is bottleable or not. But chemistry is more than the synthesis of new molecules.
Theoretical analysis of aromaticity in benzene and its analogs has shown a specific electronic component that comprises the number of π electrons that are connected in a peculiar way to make some organic molecules more stable and less reactive than one would expect. Boldyrev and Wang have picked up this concept and show that a similar aspect is found in some inorganic compounds and that it can even be extended to species where the atoms are bonded through σ orbitals.
The stability of these molecules came to the fore by mass spectrometric investigations showing that in these systems σ electrons obey similar rules as π electrons. These experimental and theoretical investigations give us valuable information about the stability patterns of substances. This may not be interesting for people who want to put compounds into bottles, but it is highly relevant for people who are using chemistry as a science for providing information and understanding of the nature of matter.
At the center of the controversy over the use of the term aromaticity is Hoffmann’s viewpoint that bottleable compounds are more important in chemistry than molecules having only a fleeting existence. This is the standpoint of orthodox chemists, which can be explained by considering the way chemistry evolved as a science, namely with the astonishing ability to make new substances that have proven to be useful for mankind. Chemical research created a new industry carrying its name, arguably the most important industrial sector.
But chemistry is more than just the collection of bottleable compounds! It is the science of understanding the material world on a molecular scale. For example, the 1999 Nobel Prize in Chemistry went to Ahmed Zewail for elementary studies on transition states of chemical reactions. This significantly broadened our knowledge about reaction pathways, but the relevance for synthetic chemistry is not obvious
I want to emphasize that many new developments often begin from rather exotic observations that were not considered to be very relevant. I want to remind everyone of the fullerenes, which in the beginning were found because of a peculiar mass spectrometric signal. That discovery evolved into a very interesting and important new class of compounds that is nowadays the focus of much experimental work, including compounds that are bottleable. Even if that had not been the case, this fascinating species would be very important for chemistry. The same holds true for Boldyrev and Wang’s planar Al42–and B9− species.
Miquel Solá
Miquel Solá, University of Girona, Catalonia, Spain, conducts theoretical studies analyzing electron delocalization and aromaticity in organic and inorganic species.

From the beginning, aromaticity has been associated with kinetically and thermodynamically stable compounds. However, the concept is in constant evolution. Over time, a remarkable expansion has occurred in the number of different types of aromatic systems known and in our understanding of aromaticity. Newly discovered aromatic molecules include the metal clusters Al42– and Ta3O3– by Alexander I. Boldyrev, Lai-Sheng Wang, and coworkers and the Möbius aromatic hydrocarbons by Rainer Herges and coworkers.
The field of aromaticity has been enriched––and not cheapened, as Roald Hoffmann suggests––by all these fascinating new aromatic compounds. We now recognize that the aromaticity concept can be applied to the entire periodic table. There is no longer a unique type of aromaticity––that is, classical π-aromaticity. Compounds can also have σ-, δ-, and even ϕ-aromaticity (involving s-, d-, and f-orbital electrons), together with combinations of these different types (multifold aromaticity).
Hoffmann connects the concept of aromaticity with stability: “Aromatic molecules are ‘bottleable,’ ” he says. Yes, the link between aromaticity and stability is well established. It is not always the case, however, that among a series of isomers or electronic states that the most stable is the most aromatic. Aromaticity is one of many factors that affect the relative energies of isomers. Other aspects such as strain energy, hyperconjugation, the presence of hydrogen bonds, long-range interactions, and so forth may have, in some cases, a greater influence.
One interesting example concerns the o-, m-, and p-benzyne species, the three possible biradicals generated by removing two hydrogen atoms from benzene. For these molecules, the order of stability is o-benzyne > m-benzyne > p-benzyne, whereas the aromaticity order of the biradicals is exactly opposite: p-benzyne > m-benzyne > o-benzyne.
Given that among the approximately 20 million compounds known, two-thirds are fully or partially aromatic, it is clear that many chemical reactions are influenced by the increase or decrease in aromaticity all across the reaction pathway. An important group of these kinds of reactions are the pericyclic ones, such as Diels-Alder cycloadditions, for which Hoffmann is famous (Woodward-Hoffmann rules). Diels-Alder reactions are now well-known to take place through aromatic transition states. The transition state is the less stable species and obviously a nonbottleable species along the reaction path. But its aromaticity, which is widely accepted by the chemical community, is responsible in part for the relatively low barrier of this cycloaddition.
In 1985, when fullerenes were discovered, they were nonbottleable molecules. However, that status changed in 1991 when Wolfgang Krätschmer and coworkers found a method that allowed the preparation of macroscopic quantities of C60. In the future, we could have the same situation for some of the all-metal clusters detected by Boldyrev and Wang.
I am much in favor of Boldyrev and Wang’s position. We have to admit, however, that indiscriminate use of newer computational methods to define aromaticity has introduced some hype. A number of cases exist in which these measures provided wrong aromaticity assignments. For this reason, it is usually recommended that aromaticity be analyzed by employing a set of aromaticity descriptors of different origin: structure-based, magnetic-based, and electronic-based indexes. It is safe to conclude that a system is aromatic only if most or all of the indexes used point in that direction.
Dage Sundholm
Advertisement
Dage Sundholm, University of Helsinki, Finland, develops quantum chemistry methods to study aromaticity by calculating magnetically induced current densities.
In Roald Hoffmann’s article, he suggests that aromaticity should be reserved for bottleable molecules. Thus, he wants to maintain a traditional view of aromaticity and questions whether molecular species such as Al42– should be called aromatic.
Alexander I. Boldyrev and Lai-Sheng Wang disagree. Their notion is supported in a Chemical Reviews article by Alexandru T. Balaban, Paul von Ragué Schleyer, and Henry S. Rzepa in which they begin the introduction by stating that “aromaticity can be extended equally well to inorganic or organometallic chemistry.”
I personally do not care whether Al42– can be considered aromatic or not. Aromaticity is just a name. What is more important to me is that molecules such as Al42– are formed in molecular beam experiments and how we can explain why this species is more abundant in the beam than other negatively charged aluminum clusters.
The aromaticity concept provides a useful description of the chemical bonding of the cluster. One could invent a new name, but why? The original aromaticity concept as used in organic chemistry is not very exciting these days, as compared with modern aromaticity views. Traditional aromaticity has perhaps played out its role because we more or less understood how it works.
Chemical concepts evolve. Therefore if we restrict the aromaticity discussion to well-known aromatic, antiaromatic, and nonaromatic organic molecules, then what shall we do with novel aromaticity concepts such as spherical aromaticity, cylindrical aromaticity, homoaromaticity, Möbius aromaticity, aromaticity of triplet states, and more? These are all extensions of Hückel 4n + 2 aromaticity originating from August Kekulé’s idea, as Hoffmann explains. I do not find any reason for excluding another kind of aromaticity if the concepts are useful. It is probably better to welcome them into the realm of aromaticity than to create new names for them.
Novel methods to assess the degree of aromaticity have also been developed. Hoffmann mentions Schleyer’s nucleus-independent chemical shift method, energy stabilization, lack of bond-length variations, reactivity, and nuclear magnetic resonance chemical shifts. I would like to add my work on calculated values for magnetically induced ring-current susceptibilities. By comparing ring-current susceptibilities of different molecules, one can determine the degree of electron mobility around the ring. I relate the electron-delocalization and electron mobility properties to the degree of aromaticity because then chemists will understand what I mean.
I agree there is aromaticity hype. However, that will disappear in the future when we have invented better and more reliable methods to determine the degree of aromaticity, or whatever we agree to call it.











Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter