Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
New Drug Approvals Soar In 2014
Pharmaceuticals: FDA approvals reached 18-year high last year
by Lisa M. Jarvis
January 6, 2015
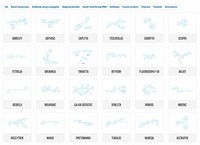
In an encouraging sign of the health of the pharmaceutical industry, new drug approvals soared to an 18-year high in 2014. The Food & Drug Administration gave the green light to 41 new molecular entities last year, up from 27 in 2013.
In years past, a surge in new approvals often meant a flood of me-too drugs had made it onto the market. But the 2014 class of new drugs is ripe with innovation: The list includes 16 first-in-class treatments, compared with just nine drugs with a novel mechanism of action approved in 2013.
Last year was the best year ever for rare disease drug approvals, FDA said, with orphan drugs accounting for roughly 40% of the new drugs approved. Other highlights included 12 new treatments for infectious diseases, including four much-needed new antibiotics. And eight new cancer drugs hit the market, a crop that included several immunotherapies that represent major advances for patients.
FDA crammed seven of those new drug approvals into the last month of the year. The final push included several key products: Amgen’s bispecific antibody Blincyto, for the treatment of a type of leukemia; AbbVie’s Viekira Pak, a combination of antivirals for the treatment of hepatitis C; and Bristol-Myers Squibb’s melanoma immunotherapy Opdivo.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter