Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Development
FDA gives its nod to 53 new drugs in 2020
Despite a pandemic, a steady flow of new molecular entities reached the market last year
by Lisa M. Jarvis
January 4, 2021
| A version of this story appeared in
Volume 99, Issue 2
The US Food and Drug Administration kept up a blistering pace of new drug approvals last year, all while navigating the urgent evaluation of tests, drugs, and vaccines in development for COVID-19. The agency approved 53 new products in 2020, the second-highest number in more than 20 years.
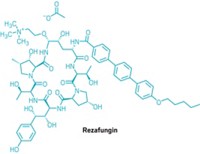
Of those new molecular entities, 35 were small molecules—which at 66% of the total approvals continue to be a critical component of the drug pipeline. Among the notable approvals: two treatments for Ebola virus, the first fully-approved antiviral for COVID-19, the first oral treatment for spinal muscular atrophy, and the first treatment for children with a rare genetic disease that causes them to develop tumors on their nerves. The year also brought 10 new cancer treatments, including another 2 antibody-drug conjugates, a class that has become an increasingly permanent fixture in the new drug list.
Steady flow
Click here or the image below to view the full interactive table.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter